ANTIPSYCHOTIC TREATMENT AND PSYCHOSOCIAL FUNCTIONING IN SCHIZOPHRENIA – RESULTS FROM ROMANIAN COHORT OF EUFEST STUDY
Abstract
Introduction: Schizophrenia represents a chronic and debilitating illness listed by the international organisms as top priority for public health actions to reduce disability and improve the function and quality of life for people living with chronic illness. Objective: The aim of this study was to compare psychosocial functioning of schizophrenic patients after 1 year of neuroleptic treatment in a first episode schizophrenia population treated with atypical or typical antipsychotics. Methods: Procedures of the European First Episode Schizophrenia Trial study (EUFEST) have been previously described. Data analyzed in this paper refers to Romanian patients (N = 113) included in the EUFEST study. Psychosocial functioning was assessed by Global Assessment of Functioning (GAF). GAF questionnaire was applied at baseline and at 1, 2, 3, 6, 9 and 12 months (end of study). Results: Mean value at 1 year compared with baseline was 76.88 (±16.20) vs. 39.06 (±13.56) for Haloperidol, 75.58 (±18.41) vs. 40.67 (±12.71) for Olanzapine, 78.57 (±10.15) vs. 37.65 (±17.05) for Quetiapine, 82.35 (±10.16) vs. 38.24 (±12.52) for Amisulpride, 76.11 (±9.47) vs. 38.33 (±10.26) for Ziprasidone. Statistical comparison with ANOVA revealed no statistically significant differences, p = 0.533. The improvement in functionality in patients did not differ between antipsychotics from one evaluation to another. During the antipsychotic treatment, the psychosocial functioning of patients improved significantly more during the first 6 months, irrespective of treatment arms. After that, the improvement continued to develop, but at a slower pace. Conclusions: Our study didn't support the superiority of atypical neuroleptics versus classical ones regarding functionality in first episode schizophrenic patients. The improvement starts fast with both classes of drugs and the larger part of improvement in functionality took part during the first 3−6 months of treatment.
INTRODUCTION
All chronic illnesses have the tendency to limit or even decrease the functional status of people that live with them, which is reflected in all life domains: psychological (accurate understanding and interpretation of people’s behavior), social (performing a social role), occupational (capacity to perform obligations at workplace, school and so on), leisure (capacity to enjoy spending time doing things that you like). Therefore the quest to achieve a better living for people having a chronic disease, it is of paramount importance and is in line with the current healthy-longevity promotion (1). Schizophrenia, dementia and depression represent chronic and debilitating illnesses listed by the international organisms as top priority for public health actions to reduce disability and improve the function and quality of life for people living with chronic illness (2).
Schizophrenia is a heterogeneous clinical syndrome, i n c l u d i n g p o s i t i v e a n d n e g a t i v e f e a t u r e s a n d neuropsychological impairment, also having impaired social and occupational functioning as a core characteristic.
There is an important debate about how much each dimension contributes to functionality of schizophrenia patients with the bulk of the data (3, 4, 5) suggesting that cognitive and negative symptoms decisively participate in long term functionality of schizophrenic patients. Both Kraepelin and Bleuler were aware that positive symptoms do not represent the core of schizophrenia. Of course treating positive symptoms still represents an important part of the management of schizophrenia, but by no means equals the most important part. During the acute phase of schizophrenia the patient may act in way which can be extremely deleterious for his social, professional or familial relationships not to mention direct threats of personal health or even life (i.e. during an acute exacerbation of symptoms one patient having command auditory hallucinations may act on them and kill himself etc., etc).
Reduction of positive symptoms has been therefore an important goal in schizophrenia therapy since the beginnings of drug treatment developments, despite the fact that very soon clinicians became painfully aware of the importance of treating negative and cognitive symptoms, as key goals for a better functioning of the patients.
Guidelines for treatment in schizophrenia recommend that primary treatment should be pharmacological, antipsychotics representing the mainstay of the treatment (6). Antipsychotics are currently classified into: first generation, conventional or ‘typical’ antipsychotics (FGAs), second generation ‘atypical’ antipsychotics (SGAs) (7). Both classes of antipsychotics are targeting positive symptoms in schizophrenia and are capable of inducing extrapyramidal symptoms (EPS), however, atypical antipsychotics have some evidences regarding efficacy on negative and cognitive symptoms (8), although the mean effect is recognized to be small (9, 10) and are known for inducing less EPS but instead are associated with an increased risk for metabolic syndrome (11).
Recent studies challenge (12,13), the initial optimism that atypical antipsychotics are indeed associated with an improvement in negative or cognitive symptoms despite the fact that are authors who consider that a superiority in functionality in the case of patients treated with atypical antipsychotics compared with patients treated with conventional antipsychotics appear after a longer time of treatment (14).
Future research should aim to determine the most feasible and valid measure of functional status in schizophrenia, as we set as a target for treatment functional recovery. Efficacy of antipsychotics has been quantified by complex analyses of scores in scales of symptoms reduction, like PANSS (15, 16), but also by all-cause treatment discontinuation – the EUFEST and CATIE studies (13, 17), and quality of life – the CUtLASS study (18).
The most important goal of antipsychotic treatment in psychotic diseases is functional recovery (19). Assessment of functional status might represent a better indicator of overall status of a certain schizophrenic patient than psychometric tests that assess symptoms. Psychosocial functioning is strongly correlated with cognition and negative symptoms, but these domains are currently poorly targeted by schizophrenia treatment. The development of atypical antipsychotics was considered a very important step in the treatment of schizophrenic patients and a great hope at that moment also for i m p r o v i n g p s y c h o s o c i a l f u n c t i o n i n g . A t y p i c a l antipsychotics were created and proved to have less EPS and sedation which could have made instrumental daily activities easier; also improvement on negative symptoms could have made the patient to regain interest and initiative.
However, the results of later studies weren’t so optimistic. The latest data comparing typical and atypical neuroleptics in schizophrenia treatment didn’t reveal important differences between the 2 classes of antipsychotics regarding efficacy in improving cognitive and negative symptoms (9, 13).
Clinical trials mostly assess efficacy (reduction of symptoms), which is less easy extrapolated to real life. In order to clearly define benefits of antipsychotics in treatment of schizophrenia, a study should assess effectiveness or achievement of functional outcomes, since this could make the study more real-life oriented. There are still scarce data on effectiveness of schizophrenia treatments.
Most clinical trials have used for establishing efficacy of an antipsychotic and subsequent marketing have included a selected patient population, too few women and subjects with comorbid conditions, which may not be a good reflection of patients seen in everyday practice (20). In order to overcome these drawbacks, large project trials like CATIE, Cafe, SOHO, CUtLASS, EUFEST have been designed in a naturalistic manner, in order to assess also effectiveness of antipsychotics, which means efficacy plus tolerability (21).
EUFEST trial is one of the most valuable naturalistic studies which assess effectiveness of antipsychotics in recovery of functional status because it includes first episode and treatment naive patients. EUFEST study has used DSM IV axis V evaluation of functionality – the Global Assessment of Functioning (GAF) scale (22). Although in present, the DSM V is recommending use of WHODAS as a global measure of disability in psychiatric disorders, the use of GAF has been proven throughout a decade of studies a simple and reliable measure of functional status (23).
Attitudes towards schizophrenia treatment have changed in view of recent neurobehavioral and epidemiological researches, which are showing that action directed towards improving functional status can have a profound impact on its onset and outcome (24). By assessing and evaluating psychosocial functioning in schizophrenic patients, it may be possible to determine the clinical status of a patient under treatment with antipsychotics.
To address this issues, we analyzed data from EUFEST study (Romanian patients) relating to functional status of patients with first episode schizophrenia after 1 year of antipsychotic treatment. We also compared the efficacy of treatment on functional outcome across classes of drugs, between atypical and typical antipsychotics.
METHODS
Data analyzed in our paper are drawn from European First Episode Schizophrenia Trial study (EUFEST).
The purpose of EUFEST study (25) was to compare atypical antipsychotics (amisulpride 200–800 mg/d, olanzapine 5–20 mg/d, quetiapine 200–750 mg/d, ziprasidone 40–160 mg/d) with low doses of typical neuroleptics (haloperidol 1–4 mg/d) in terms of effectiveness. The measure for effectiveness in EUFEST is all-cause treatment discontinuation, defined as the time to discontinuation of the study drug to which patients were originally randomized.
Investigators from 50 centers in Europe and Israel participated into this trial. Eligible patients were 18– 40 years of age and met DSM- IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder confirmed by the Mini International Neuropsychiatric Interview Plus.
Inclusion criteria were: 18– 40 years of age and DSM- IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder confirmed by the Mini International Neuropsychiatric Interview Plus, first episode of disease with no more than 2 years elapsed between the onset of positive symptoms and recruitment into the trial and previous use of antipsychotic drugs of less than 2 weeks during the preceding year and less than 6 weeks lifetime.
The investigators invited eligible patients to participate providing information orally and in writing about the trial. After complete description of the study to the subjects, written informed consent was obtained. The trial complied with the Declaration of Helsinki and was approved by the Ethics Committees of the participating centres.
Patients did not meet exclusion criteria in this study if: no more than 2 years elapsed since the onset of positive symptoms; any antipsychotic have not been used exceeding 2 weeks in the previous year or 6 weeks lifetime; patients had not a known intolerance to one of the study drugs; and patients didn’t meet any of the contraindications for any of the study drugs as mentioned in the (local) package insert texts.
Baseline data were obtained between 4 weeks before and 1 week after randomization on demographics, diagnoses, current medication, psychopathology (Positive and Negative Syndrome Scale – PANSS), severity of illness (clinical global impression – CGI), overall psychosocial functioning (global assessment of functioning scale – GAF), extrapyramidal symptoms (St Hans rating scale – SHRS), depression (Calgary Depression Schizophrenia Scale – CDSS), neurocognitive performance (six measures: Trail Making A [time], Flexibility Index [Trail Making B–A time], Wechsler Adult Intelligence Scale Digit-symbol Coding [total correct], Purdue pegboard [total pegs with dominant hand], Rey Auditory Verbal Learning Test – learning index [total correct on trials I–V], and Rey Auditory Verbal Learning Test – secondary memory [total correct on Delayed Recall trial]), and quality of life (Manchester Short Assessment of Quality of Life – MANSA).
All scales have been given to the included patients at Baseline, 1, 2, 3, 6, 9, and 12 months. Data about quality of life were evaluated at baseline, month 3 and end of study (month 12).
In this article we used the term ‘schizophrenia’ for diagnostics of schizophrenia, schizophreniform and schizoaffective disorder.
Data analyzed in my paper refer to Romanians patients (N=113) included in this study.
STATISTICALANALYSIS
We u s e d d e s c r i p t i v e a n a l y s i s t o c h a r a c t e r i z e demographics and repartition into treatment arms. Mean, SD, and sample size are provided for continuous variables. Discrete variables are described using frequencies and percentages. All statistical tests were 2 tailed; α (level of significance) was 5%. Discrete variables were analyzed with a χ2 test. The differences between different treatment arms (Haloperidol, Olanzapine, Amisulpride, Quetiapine, Ziprasidone) were assessed using ANOVA function and corrected with Bonferonni t- tests. Within-group improvement in GAF scores over time was evaluated using paired-sample t tests.
Concerning cognitive functioning, for simplicity a composite score of cognitive functioning was created, after standardizing to create z scores and obtaining the mean for standardized values on the neurocognitive tests. Data were analysed using Statistical Package for Social Sciences (SPSS) version 16.
RESULTS
The EUFEST study in Romania included 113 patients which were randomized to one of the treatment arms. From the 113 initial randomized, 61 (54%) are women and 52 (46%) men.
GAF scale was applied by clinicians at baseline, 1, 2, 3, 6, 9, and 12 month (end of study).
Of the 113 patients in Romanian cohort of EUFEST study, 2 patients did not have valid baseline data and 20 patients did not complete the study. We analysed socio- demographical and clinical characteristics differences between completers and non-completers, because people who didn’t finish the study might be different (and therefore could reduce the reliability of results) from people who completed the study. We found that non- completers were more frequently (significantly statistic) having an occupation and reported a better Quality of Life (significantly statistic) comparing with completers, without any other differences. Data are presented in table 1.
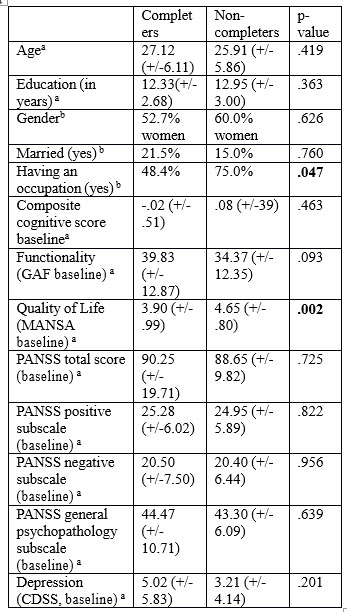
aStatistic = t-test
bStatistic= chi-square test
Table 1. Socio-demographical and clinical characteristics of completers vs non-completers in EUFEST study – Romanian cohort
There are no differences regarding treatment arm allocation between groups as evaluated by logistic regression (p=.432).
A summary of data upon socio-demographics and clinical characteristics (PANSS total score) across treatment arms in the population analysed in this study revealed no baseline differences (Table 2a and 2b).
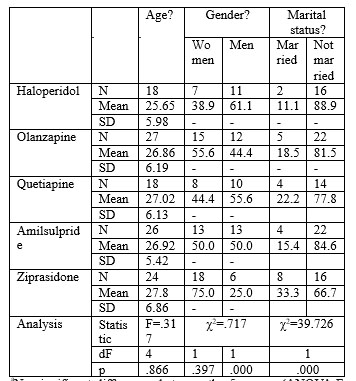
aNo significant differences between the 5 groups (ANOVA F, p>0.05) bPercentage calculated based on the total sample at baseline cNo significant differences between the 5 groups (χ2, p>0.05) dSignificant differences between the 5 groups (χ2, p<0.05)
Table 2.a. Socio-demographical data and repartition into treatment arms in EUFEST study –Romanian cohort
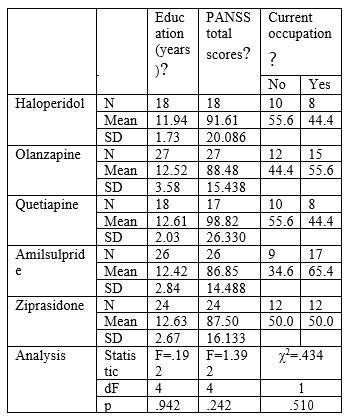
aNo significant differences between the 5 groups (ANOVA F, p>0.05)
bPercentage calculated based on the total sample at baseline cNo significant differences between the 5 groups (χ2, p>0.05) dSignificant differences between the 5 groups (χ2, p<0.05) Table 2.b. Socio-demographical data and repartition into treatment arms in EUFEST study –Romanian cohort Data about GAF scores during the study by treatment arms are presented in table 3. There was no overall difference between the five treatment groups in GAF scores between baseline and end of study time (F=.793, df=4, 92, p=0.533) or in-between visits (all p values >0.05).
Change in GAF score from baseline to end of study (12 months) showed improvement in each of the five treatment groups (Paired t-tests: haloperidol: t=-8.388, df=16, p<0.001; olanzapine: t=-8.403, df=23, p<0.001; quetiapine: t=-7.128, df=12, p<0.001; amisulpride: t=-
13.974, df=19, p<0.001; ziprasidone: t=-11.560, df=17, p<0.001).
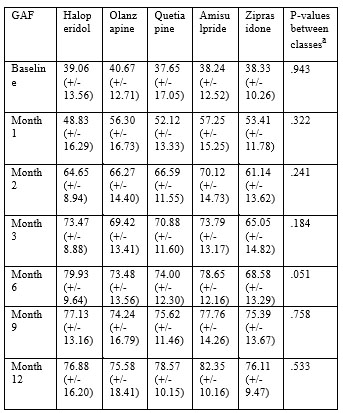
Table 3. GAF scores across treatment arms in EUFEST
study – Romanian cohort.
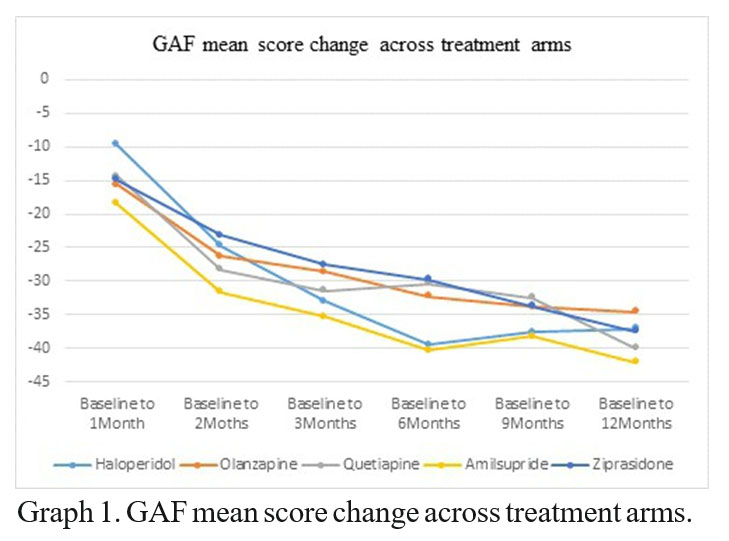
The improvement in functionality in patients is presented in graph 1. We did not note any differences between treatment arms regarding functionality in patients at the moment of inclusion in study. No significant differences between treatment arms regarding functionality occurred at any following evaluation (p>0.05 at month 1, 2, 3, 6, 9, and 12). Controlling for multiple comparisons using Bonferonni corrections t tests comparing the change in GAF score from baseline to each of the visits for haloperidol versus the other four treatment arms of second-generation antipsychotic drugs (Olanzapine, Amisulpride, Quetiapine and Ziprasidone) did not reveal any significant differences. Moreover, there are no d i ff e r e n c e s i n f u n c t i o n a l i t y b e t w e e n a t y p i c a l antipsychotics.
During the treatment, the functionality of patients greatly improved during the first 6 months irrespective of treatment arms, as it can be seen on the graphic 1 and table 4. The improvement in functionality had occurred faster in the first 3 months of treatment irrespective of treatment arm. After that, the improvement continued to develop but in slower pace
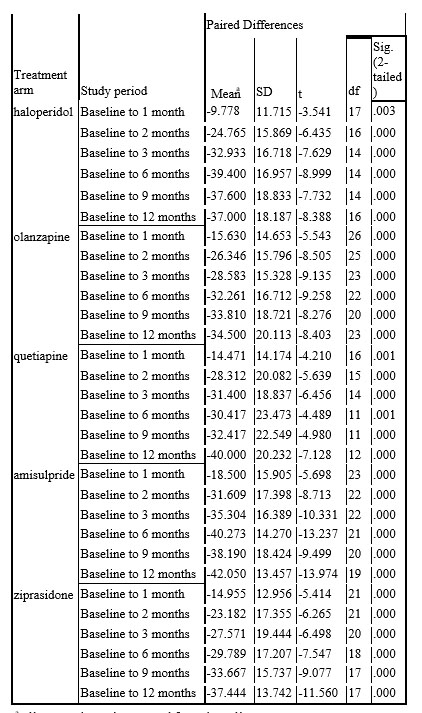
a all scores have increased from baseline aStatistic=ANOVA analysis
Table 4. GAF mean score change across treatment arms in EUFEST study – Romanian cohort
DISCUSSION
We have found that the largest part of improvement in psychosocial functioning in first episode schizophrenic patients appeared during the 6 months, afterward the improvement continued, but at a slower pace. Moreover, from the initial greater improvement (6 months) the greatest improvement took part during the first 3 months.
It would be interesting to observe if this trend continues over longer time because, if this would be the case, psychosocial functioning improvement in the first 3-6 months may represent a good predictor of long term treatment in schizophrenia. If longer studies confirm this trend, a clinical decision about which neuroleptic to use for long term may be improved by information of such type.
One limitation of our study is the fact that data are obtained about first episode schizophrenic patients. In this kind of patients, the clinical response and consequently the functionality is larger than in chronic schizophrenic patients (26, 27). Other limitation is the relatively short duration of treatment (1 year). Longer studies would be more informative regarding long term functional outcome in patients treated with older versus newer antipsychotics. Functionality of a schizophrenic patient depends on multiple characteristics mainly (but not limited at) p r e m o r b i d f u n c t i o n i n g , c o g n i t i v e f u n c t i o n i n g , psychopathology and so on. The results of our study suggest several observations:
1. Our study didn’t support the superiority of atypical neuroleptics versus classical ones regarding functionality, which may represent a larger and better suited item for clinical evolution. There is a lot of research done to prove differences between older (typical neuroleptics) and recent neuroleptics. The discussion about putative superiority of atypical neuroleptics versus the typical ones dates already for a decade (9). Recent data about clinical advantage of atypical vs typical neuroleptic seem to indicate that there are no important differences between the 2 classes (28, 29). However, longer studies are needed to validate or refute these observations.
2. The bulk of improvement of functionality in first episode schizophrenic patients appeared during the first 6 months, afterward the improvement continues, but on a slower pace. Moreover, from the initial greater improvement (6 months) the greatest improvement took part during the first 3 months. This may represent a good indicator about long term clinical evolution of patients treated with that specific antipsychotic and to guide long term treatment in schizophrenic patients.
One limitation of our study is the fact that data are obtained about first episode schizophrenic patients. In this kind of patients, the clinical response and consequently the functionality is larger than in chronic schizophrenic patients (26). Other limitation is the relatively short duration of treatment (1 year). Longer studies would be more informative regarding long term functionality in patients treated with older versus newer antipsychotics.
CONCLUSIONS
In first episode schizophrenic patients we did not found statistically significant differences regarding functionality after 1 year of treatment between typical neuroleptics and atypical neuroleptics (without differences at 1, 2, 3, 6, 9 and 12 months). The improvement starts fast with both classes of drugs and the larger part of improvement in functionality took part during the first 3-6 months of treatment. The assessment of functionality in first episode patient should be used in studies with longer duration in order to investigate which antipsychotic is best for long term use.
REFERENCES
1.Riga S, Riga D, Mihailescu A, et al. Longevity health sciences and mental health as future medicine. Ann NYAcad Sci. 2010;1197:184-7.
2.Living-Well-with-Chronic-Illness. (2011). Retrieved 2014, from w w w . i o m . e d u : h t t p : / / w w w . i o m . e d u / ~ / m e d i a / F i l e s / R e p o r t % 2 0 F i l e s / 2 0 1 2 / L i v i n g – W e l l – w i t h – C h r o n i c – Illness/livingwell_chronicillness_reportbrief.pdf
3.Twamley EW, Doshi RR, Nayak GV et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry 2002;159(12):2013-2020.
4.Swartz MS, Perkins DO, Stroup TS et al. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry 2007;164:428-436.
5.Tandberg M, Sundet K, Andreassen OA, Melle I, Ueland T. Occupational functioning, symptoms and neurocognition in patients with psychotic disorders: investigating subgroups based on social security status. Soc Psychiatr Epidemiol 2013, 48: 863–874.
6.National Institute for Health and Care Excellence. CG178 Psychosis and schizophrenia in adults: treatment and management. 2014
7.Gründer G, Hippius H & Carlsson A. The ‘atypicality’ of antipsychotics: a concept re-examined and re-defined. Nat Rev Drug Discov 2009;8(3):197-202.
8.The Collaborative Working Group on Clinical Trial Evaluations. Assessing the effects of atypical antipsychotics on negative symptoms. J Clin Psychiatry 1998;59 Suppl 12:28-34.
9.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Br Medical J 2000;321(7273):1371-6.
10.Meltzer H, Kostakaglu E, Lee M. Response. Negative Symptoms
R e d u x – L e t t e r s t o t h e E d i t o r. N e u r o p s y c h o p h a r m a c o l o g y 2000;22(6):662–664.
11.Miller R. Mechanisms of Action of Antipsychotic Drugs of Different Classes, Refractoriness to Therapeutic Effects of Classical Neuroleptics, and Individual Variation in Sensitivity to their Actions: PART I. Curr Neuropharmacol. 2009;7(4):302-314.
12.Lieberman JA, Stroup TS. et al. Clinical Antipsychotic Trials ofIntervention Effectiveness (CATIE) Investigators Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;22(353):1209-23.
13.Kahn R, Fleischhacker WW, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008;371(9618):1085-1097.
14.Tran PV, Dellva MA, Tollefson GD, et al. Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry 1998;172:499–505.
15.Fleischhacker WW & Kemmler, G. The clinical relevance of p e r c e n t a g e i m p r o v e m e n t s o n t h e P A N S S s c o r e .Neuropsychopharmacology 2007;32(11):2435–2436.
16.Leucht S, Davis JM & Engel RR. Defining “response” in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology 2007;32(9): 1903-1910.
17.Stroup TS, McEvoy JP, Swartz MS. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development.l. Schizophr Bul 2003;29:15-31.
18.Jones PB, Barnes TR, Davies L. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006;63(10):1079–1087.
19.Bowie CR, Twamley EW, Anderson H et al. Self-assessment of functional status in schizophrenia. J Psychiatr Res 2007;41(12):1012- 1018.
20.Hummer M, Fleischhacker WW. Do phase III trials have clinical value? J Clin Psychopharmacol 1999;19:391–392.
21.Agius M, Davis A, Gilhooley M, Chapman S & Zaman R. What do large scale studies of medication in schizophrenia add to our management strategies? Psychiatria Danubina 2010;22(2):323–328.
22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Washington, DC: American Psychiatric Press. 2000:34
23.Aas IH. Guidelines for rating Global Assessment of Functioning (GAF). Annals of General Psychiatry 2011;10:2.
24.Brissos S, Molodynsky A et al. The importance of measuring psychosocial functioning in schizophrenia. Annals of Gen Psychiatry 2011;10:18.
25.Fleischhacker WW, Keet IPM, Kahn RS. The European First Episode Schizophrenia Trial (EUFEST): Rationale and design of the trial. Schizophrenia Res 2005;78:147– 156.
26.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev 2011;35:573-588.
27.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive Values of Neurocognition and Negative Symptoms on Functional Outcome in Schizophrenia: A Longitudinal First-Episode Study With 7-Year Follow- Up. Am J Psychiatry 2005;162:495-506.
28.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012;379(9831):2063–2071.
29.Swartz M, Perkins DO, Stroup TS et al. Special Section on Implications of CATIE: What CATIE Found: Results From the Schizophrenia Trial. Psychiatric Services 2008;59(5):500-506.
***




