DEPRESSION IN YOUNG ADULTS WITH CHRONIC SOMATIC ILLNESS – AN ANALYSIS OF 1970 BRITISH COHORT STUDY
Abstract
The prevalence rate of depression occurring in people with somatic illnesses is 3 times more than people without somatic illnesses, but less research was conducted in this respect for people with early onset of somatic disease. Taking into account that the presence of depression in chronic patients aggravates the somatic disease, leading to a poor prognosis and higher rates of mortality it is important to detect and treat it. The purposes of our study are to analyse in a large cohort (BCS-70) the risk of depression in patients with somatic illness at early ages (less or equal 30 years). Methods Data used in our paper were drawn from the 1970 British Cohort Study (BCS70). The design and conduct of this study have been described elsewhere (1). Data for the present paper are drawn from 30 years wave. The data were analysed with descriptive function, chi-square and logistic regression. All analyses were performed using SPSS 16. Results At age 30, we have found 1409 people (12.7%) with depression. From a total of 11211 people there are 860 people with hypertension (7.7%) and 109 people with diabetes (1%). There is an increased risk of depression in the case of people with „pure” hypertension or „pure” diabetes (p<0.05). In the case of people with both hypertension and diabetes, logistic regression showed that hypertension is a risk factor for depression irrespective of socio-economic status and diabetes (Exp(B)=1.970, CI=1.579-2.458, p=.001). Conclusion The above data indicate that risk of depression is higher in people with onset before 30 years old of hypertension and /or diabetes. Our study did not identify an increased risk for depression by socio economic class. Data of our study clearly suggests that it is extremely important that young patients with somatic diseases like diabetes and/or hypertension should be aggressively screened and treated for depression. 1. Butler NR, Golding J, Howlett BC. From Birth to Five: A Study of the Health and Behaviour of a National Cohort. Oxford: Pergamon,1985. Bynner J, Butler N, Ferri E, et al. The design and conduct of the 1999–2000 surveys of the National Child Development Study and the 1970 British Cohort Study. UK Data Archive In: CLS Cohort Studies Working Paper 1. London: Centre for Longitudinal Studies, Institute of Education, University of London, 2000.
Introduction
Depression represents a major cause of morbidity and mortality (1). The prevalence of depression is increasing (2) and by 2012 it is reported to be among the first 10 diseases causing loss of years due to disability (3). Depression is responsible for the greatest proportion of burden associated with non-fatal health outcomes accounting for approximately 10% total years lived with disability(4), more than cardiovascular disorders (2.8%), diabetes (3.0%), or chronic respiratory diseases (6.3%)(3). Chronic somatic illnesses are the cause of significant burden on individuals, families, societies and countries. Chronic somatic diseases that are frequent in the age group 18-44 years old are cardiovascular disease (most frequent hypertension – reported frequencies between 1.4%(5) and 8.7% (6)), cancer (aprox 2% prevalence (7)), diabetes (reported frequencies 1.4%(5) to 2.6%(8)) and respiratory diseases (aprox 4%(5)).
The most frequent chronic medical conditions associated with depression are: heart disease(9,10), hypertension(11), diabetes (12,13), asthma (14). The prevalence rates of depression occurring in people with somatic illnesses is two (15) to three (16) times more than people without somatic illnesses. In patients with two or more chronic medical conditions the risk of depression is six times higher comparing with healthy persons(17).
Many studies focused on co-morbidity of depression with one single chronic condition. Researchers found a high prevalence of depression in people with diabetes, its rates varying by diabetes type and among developed and developing nations (18). The prevalence of depression in diabetes patients may range between 2 and almost 30% (19-23), with higher values found in patients with type 2 diabetes (24,12) and among females with diabetes (25). Prevalence in diabetic group age 18-44 years old is less than 5% (8). Young adults with diabetes face difficulties with diabetes self-management and co-morbidity with depression is deteriorating even more diabetes adaptation (26,27).
The burden of comorbidity
Morbidity and mortality in patients with somatic illnesses and depression are significantly higher than in patients with somatic illness who are not depressed. These patients face many negative health consequences: they present more severe somatic symptoms and complications (28), have poor outcomes of somatic disease and more functional disability (29), a lower quality of life (30,31), a low treatment adherence (32,33), an increased use of medical services (34,35) and higher heath care costs–(3638).
Co-morbid depression among patients with diabetes is associated with poor diabetes outcomes (such as glycaemic control), with more and greater complications (such as diabetic retinopathy, nephropathy, neuropathy) with functional disability –(3941), with a lower quality of life (23), with a decreased adherence(35) and with higher healthcare costs(42).
Another relevant example is the link between hypertension and depression. Clinical studies show that depression is common in patients with hypertension, has a certain influence in blood pressure control and a significant impact on severity of hypertension (43,44). Depressive symptoms have been found to be predictive of hypertension in young adults(45).
The association between cardiovascular illness and depression has multiple biological and clinical links and is likely bidirectional. Most cited pathophysiological mechanisms are hormonal variations, metabolic abnormalities, hypercoagulability, increased platelet aggregation, inflammation, and endothelial dysfunction (46,47). Depression is most likely aggravating the associated cardiovascular disease by impeding the optimal care for this people.
The importance of depression detection.
Although depression has a high prevalence and it is present in patients with chronic somatic illnesses, depression among these patients remains often undetected (19,37). Although depression is highly prevalent in individuals with multimorbidity, studies on the rates and correlates of depression in these individuals are scarce (48).
People aged less than 30 have a prevalence of somatic illness less than 10%, however, a chronic disease that begins early will result in a worse overall prognosis of the person affected (49). In this respect, our study is attempting to study if there is an increased risk for depression for people with two frequent somatic illnesses, hypertension and diabetes at age 30. Because the association between these two illnesses is quite common, we looked at the increasing or risk for depression not only in pure diabetes and hypertensive groups but also in N=183 N=10 N=17 increasing the risk for depression in comorbid group (i. e. hypertension and diabetes).
Methods
Data used in our paper were drawn from the 1970 British Cohort Study (BCS70). The 1970 British Cohort Study (BCS70) is a cohort study that enrolled 16 567 babies born in England, Scotland, and Wales on 5-11 April 1970 (50,51).
The original study focused on children health and has successively expanded to examine physical, educational and social development of these children. Individuals participating in this cohort study were assessed at birth with a 96.7% response rate and in ongoing follow-ups using a multi-method, multi informant approach. Participants were followed up at 5 (n = 13 135, in 1970),
10 (n = 14 875 in ), 16 (n = 11 622 in 1986), 26 (n= 9003, in
1996), 30 (n = 11 261 in 2000) and 34 (n=9656, in 2004)
years of age.
At 30 years, marked efforts were made to recruit difficult- to-reach subjects (51). Data for the present paper are drawn from 30 years wave(52).
Measure of depression. At age 30, data was collected on the severity of depressive symptoms using The Malaise Inventory. An overall Malaise score for a cohort member is the sum across the individual variables, yielding a minimum score of 0 and a maximum of 24. A score of 8 or higher is a recommended cut-off for a depressive episode (53).
Measure of hypertension and diabetes. Data about somatic illnesses were obtained from patients. At age 30 people from cohort were asked:
“Have you ever had or been told you had high blood pressure?”
“Have you ever had diabetes?”
They could respond with “yes”, “no”, “don’t know” and “not answered”. We excluded from the analysis the last 2 groups of responders.
Measure for socio-economic status. Data about social class of people from cohort were used as proxy for socio- economic status (ses). Social classes were defined as such: “Professional”, “Managerial-technical”, “Skilled non- manual”, “Skilled manual”, “Partly skilled” and “Unskilled”.
Statistical analyses. We created 2 groups, the first one comprised of people considered controls (overall Malaise score less or equal 7), the second one assumed to have depression (Malaise score of 8 or over). The data were afterward analysed with descriptive function, chi-square and logistic regression. All analyses were performed using SPSS 16.
Results.
Descriptive data. From a total of 11211 people there are860 people with hypertension (7.7%) and 109 people with diabetes (1%). From a total of 11211 people 1409 (12.6%) have depression, and from this, 193 have developed hypertension (high blood pressure) and 27 have developed also diabetes, as it is shown in the figure below.
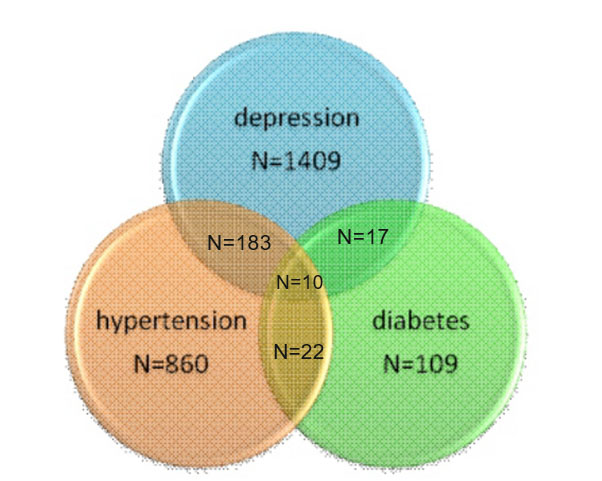
Figure 1. Distribution of hypertension, diabetes and depression within the BCL-70 cohort
Data were first analysed with chi square. The proportion of people with depression in the hypertension group is higher 21.5% (183 out of 852) than the proportion of depression in non-hypertensive group 11.9% (1225 out of 10256), OR =2.017, CI=1.694-2.400, p=.001. The proportion of people with depression in the diabetes group is also higher 24.8% (27 out of 109) than the proportion of depression in non-diabetes group 12.6 % (1382 out of 11003), OR=2.292, CI=1.478-3.554, p=.001.
Socio-economic status. It is virtually impossible to evaluate the causality in a cross-sectional study like this wave of the cohort. However, due to the fact that socio- economic status may influence hypertension, diabetes and depression, we analysed data to see this putative influence. Our analysis showed that socio-economic status did influence hypertension (Exp(B)=.879 CI=.797- .969, p=.009), depression (Exp(B)=1.288, CI=1.118- 1.398, p=.001) but not diabetes (Exp(B)=1.081, CI=.811- 1.440, p=.597).
Moreover, there is also possible that people with hypertension may be at risk for diabetes, and therefore we analysed data (chi square) and indeed, people with hypertension have a higher proportion of diabetes 3.7% (32 out of 860) comparing with people with diabetes but without hypertension .7% (77 out 10347), (OR 5.155, CI= 3.393-7.832, p=.001).
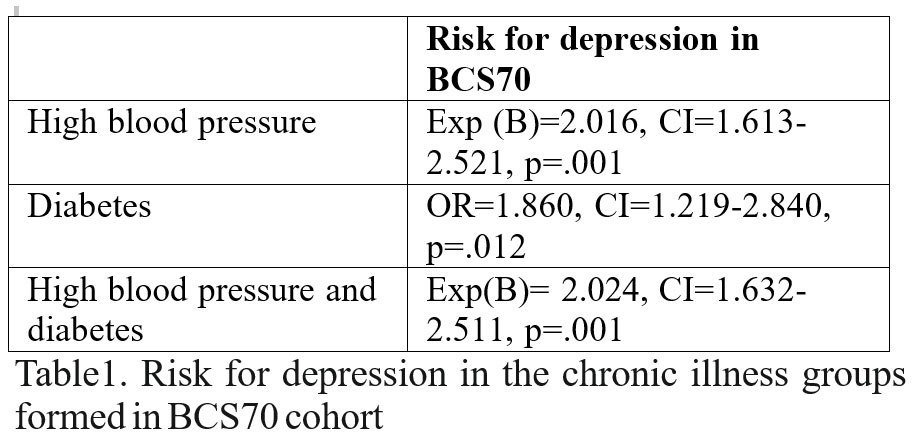
Because the possible influences between variables are intricate (socio-economic status may influence somatic illnesses but also depression, somatic illnesses may increase depression risk and depression may increase risk for somatic illnesses or decrease socio- economic status and somatic illnesses may increase the risk for the other somatic illness) we decided to analyse all the data with logistic regression using depression as dependent variable and hypertension and diabetes as independent variable, while controlling for socio- economic status. In this model depression risk is increased by hypertension (Exp(B)=1.970, CI=1.579-2.458, p=.001) and socio-economic status (Exp (B)=1.280, CI=1.180-1.389, p=.001) but it is not increased by diabetes (Exp (B)= 1.623, CI=.859-3.065, p=.135).
The above data seem to indicate that hypertension is a risk factor for depression irrespective of socio-economic status and diabetes. However, when we analysed data only for people with diabetes but without hypertension (chi square, without controlling for socio- economic status because we already seen from previous presented analyses that socio-economic status didn’t influence diabetes risk) it appeared that diabetes also increased risk for depression, 21.1% from diabetes group (17 out 77) and 11.9% (1208 out of 10179) from healthy people, p<0.05 (see table below).
We looked for risk of hypertension but without diabetes, while controlling for socio-economic status (because socio-economic status did influence, as seen from previous analyses hypertension and depression risk). There is an increased risk for depression in “pure” hypertension group (hypertension without diabetes), p<0.05 (see table below). We are also looking in group comprised of people with hypertension and diabetes while controlling for socio-economic status and there again it is an increased risk comparing with normal group, p<0.05 (see table below).
Data resulting from logistic regression are shown in the table below.
Discussion
Our study supports that both diabetes and hypertension increased depression risk, which replicates
results from previous studies –(24,13,19,48,45,5456). We would have preferred to have more objective data about the presence chronic somatic illnesses at 30 years adults. However, the only questions from the cohort which could have offered more objective data “Subject seen a doctor for high Blood Pressure in past 12 months?” and “Subject seen a doctor for diabetes in past 12 months?” come with huge numbers of missing questions (10914 for the first one and 11178 for the second one leaving to few subjects to be analysed 295 for hypertension and 78 for diabetes) and no control group, therefore impossible to analysed. The lack of more objective information regarding somatic illnesses represents one important weakness of our study.
However, strength of our study is that it looked into the association between the 2 illnesses, which is rather rare in this kind of study. Results of our study suggest that the risk is a little bit higher in co-morbid group, but the augmentation of this risk is not quite significant. Moreover, our study controlled the data for socio-economic status, which is rare in this kind of study.
One more important observation is the fact that people included in analysis are young, while other studies
of this kind looked in rather older population (49,57,58). The young age of onset of chronic illness indicates that they may be predisposed to more severe evolution. The pervasive impact of depression on quality of life and its potential negative effect on chronic disease management warrant aggressive screening and clinical interventions appropriate to each country’s healthcare system in young adults with diabetes and/or hypertension.
References
1. Michaud CM. Burden of Disease—Implications for Future Research.
JAMA. American Medical Association; 2001; Feb 7;285(5):535.
2. Andersen I, Thielen K, Bech P, Nygaard E, Diderichsen F. Increasing prevalence of depression from 2000 to 2006. Scand J Public Health.
2011; Dec;39(8):857–63.
3. WHO | Estimates for 2000–2012. World Health Organization;
4. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; Dec 15;380(9859):2163–96.
5. Carlsson AC, Wändell P, Ösby U, Zarrinkoub R, Wettermark B, Ljunggren G. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden – a challenge for public health. BMC Public Health. BioMed Central. 2013; Jan 18;13(1):670.
6. Summary Health Statistics: National Health Interview Survey [Internet]. Table A-1a. Age-adjusted percentages (with standard errors) of selected circulatory diseases among adults aged 18 and over, by selected characteristics: United States, 2014. p. 1. Available from: http://ftp. cdc.gov/pub/Health_Statis tics/NCHS/NHIS/SHS/2014_SHS_ Table_A-1.pdf
7. Cancer incidence by age | Cancer Research UK [Internet]. Available from: http://www.cancerresearchuk.org/health-professional/cancer statistics/incidence/age#heading-Four
8. Early Release of Selected Estimates Based on Data From the National Health Interview Survey, January–March 2014 [Internet]. Available from: http:// www.cdc. gov/nchs/ data/nhis/ earlyrelease/ earlyrelease201409_14.pdf
9. Versteeg H, Hoogwegt MT, Hansen TB, Pedersen SS, Zwisler A-D, Thygesen LC. Depression, not anxiety, is independently associated with
5-year hospitalizations and mortality in patients with ischemic heart disease. J Psychosom Res. 2013; Dec;75(6):518–25.
10. Kent LK, Shapiro PA. Depression and related psychological factors in heart disease. Harv Rev Psychiatry. 2009;Jan;17(6):377–88.
11. Scalco AZ, Scalco MZ, Azul JBS, Lotufo Neto F. Hypertension and depression. Clin (Saõ Paulo, Brazil). 2005; Jun;60(3):241–50.
12. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006; Dec;23(11):1165–73.
13. Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med. 2006; Apr;23(4):445–8.
14. Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for future research. Clin Pract Epidemiol Ment Health. 2005; Sep 27;1:18.
15. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet (London, England). 2007; Sep 8;370(9590):851–8.
16. Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007; Jan;29(5):409–16.
17. Sartorius N, Ustün TB, Lecrubier Y, Wittchen HU. Depression comorbid with anxiety: results from the WHO study on psychological disorders in primary health care. Br J Psychiatry Suppl. 1996; Jun;(30):38–43.
18. Egede LE, Ellis C. Diabetes and depression: Global perspectives. Diabetes Res Clin Pract. 2010; Mar;87(3):302–12.
19. Li C, Ford ES, Zhao G, Ahluwalia IB, Pearson WS, Mokdad AH. Prevalence and correlates of undiagnosed depression among U.S. adults with diabetes: the Behavioral Risk Factor Surveillance System, 2006. Diabetes Res Clin Pract. 2009; Feb;83(2):268–79.
20. Li C, Ford ES, Strine TW, Mokdad AH. Prevalence of depression among U.S. adults with diabetes: findings from the 2006 behavioral risk factor surveillance system. Diabetes Care. 2008; Jan;31(1):105–7.
21. Asghar S, Hussain A, Ali SMK, Khan AKA, Magnusson A. Prevalence of depression and diabetes: a population-based study from rural Bangladesh. Diabet Med. 2007; Aug;24(8):872–7.
22. Mier N, Bocanegra-Alonso A, Zhan D, Wang S, Stoltz SM, Acosta- Gonzalez RI, et al. Clinical depressive symptoms and diabetes in a binational border population. J Am Board Fam Med. 2008; Jan;21(3):223–33.
23. Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004; May;27(5):1066–70.
24. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001; Jun;24(6):1069–78.
25. Sotiropoulos A, Papazafiropoulou A, Apostolou O, Kokolaki A, Gikas A, Pappas S. Prevalence of depressive symptoms among non insulin treated Greek type 2 diabetic subjects. BMC Res Notes. 2008; Jan;1:101.
26. Guo J, Whittemore R, Jeon S, Grey M, Zhou Z-G, He G-P, et al. Diabetes self-management, depressive symptoms, metabolic control and satisfaction with quality of life over time in Chinese youth with type 1 diabetes. J Clin Nurs. 2015; May;24(9-10):1258–68.
27. Naughton MJ, Yi-Frazier JP, Morgan TM, Seid M, Lawrence JM, Klingensmith GJ, et al. Longitudinal associations between sex, diabetes self-care, and health-related quality of life among youth with type 1 or type 2 diabetes mellitus. J Pediatr. 2014; Jun;164(6):1376–83.e1.
28. Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007; Jan;29(2):147–55.
29. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol Psychiatry. 2003; Aug 1;54(3):177–80.
30. Noël PH, Williams JW, Unützer J, Worchel J, Lee S, Cornell J, et al. Depression and comorbid illness in elderly primary care patients: impact on multiple domains of health status and well-being. 2004; Ann Fam Med. Jan;2(6):555–62.
31. Wells KB, Sherbourne CD. Functioning and utility for current health of patients with depression or chronic medical conditions in managed, p r i m a r y c a r e p r a c t i c e s . A r c h G e n P s y c h i a t r y . 1 9 9 9 ; Oct;56(10):897–904.
32. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000; Jul 24;160(14):2101–7.
33. Lin EHB, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication a d h e r e n c e , a n d p r e v e n t i v e c a r e . D i a b e t e s C a re . 2 0 0 4 ; Sep;27(9):2154–60.
34. Sullivan M, Simon G, Spertus J, Russo J. Depression-related costs in heart failure care. Arch Intern Med. 2002; Sep 9;162(16):1860–6.
35. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000; Nov 27;160(21):3278–85.
36. Unützer J, Patrick DL, Simon G, Grembowski D, Walker E, Rutter C, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997; May 28;277(20):1618–23.
37. Na Y-M, Kim K-S, Lee K-U, Chae J-H, Kim J-H, Kim D-J, et al. The relationship between depressive symptoms in outpatients with chronic illness and health care costs. Yonsei Med J. 2007; Oct 31;48(5):787–94.
38. Hunkeler EM, Spector WD, Fireman B, Rice DP, Weisner C. Psychiatric symptoms, impaired function, and medical care costs in an HMO setting. Gen Hosp Psychiatry. 2003; Jan;25(3):178–84.
39. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000; Jul;23(7):934–42.
40. Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001; Jan;63(4):551–5.
41. de Groot M, Kushnick M, Doyle T, Merrill J, McGlynn M, Shubrook J, et al. Depression Among Adults With Diabetes: Prevalence, Impact, and Treatment Options. Diabetes Spectr. 2010; Jan 21;23(1):15–8.
42. Egede LE, Bishu KG, Walker RJ, Dismuke CE. Impact of diagnosed depression on healthcare costs in adults with and without diabetes: United States, 2004-2011. J Affect Disord. 2016; May;195:119–26.
43. Chiaie RD, Iannucci G, Paroli M, Salviati M, Caredda M, Pasquini M, et al. Symptomatic subsyndromal depression in hospitalized hypertensive patients. J Affect Disord. 2011; Dec;135(1-3):168–76.
44. Rubio-Guerra AF, Rodriguez-Lopez L, Vargas-Ayala G, Huerta- Ramirez S, Serna DC, Lozano-Nuevo JJ. Depression increases the risk for uncontrolled hypertension. Exp Clin Cardiol. 2013; Jan;18(1):10–2.
45. Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med. 2000; May 22;160(10):1495–500.
46. Lippi G, Montagnana M, Favaloro EJ, Franchini M. Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Semin Thromb Hemost. 2009; Apr;35(3):325–36.
47. Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak- -the link between depression and cardiovascular disease. Nat Rev
Cardiol. 2012; Sep;9(9):526–39.
48. Vyas A, Sambamoorthi U. Multimorbidity and depression treatment. Gen Hosp Psychiatry. 2011; Jan;33(3):238–45.
49. Filipcić I, Popović-Grle S, Marcinko D, Basić S, Hotujac L, Pavicić F, et al. Screening for depression disorders in patients with chronic somatic illness. Coll Antropol. 2007; Mar;31(1):139–43.
50. Butler N, Golding J, Howlett B. From Birth to Five: A Study of the Health and Behaviour of a National Cohort. Oxford: Pergamon; 1985.
51. Bynner J, Butler N, Ferri E, Shepherd P, Smith K. The Design and Conduct of the 1999–2000 Surveys of the National Child Development Study and the 1970 British Birth Cohort Study.CLS Cohort Studies Working Paper 1. [Internet]. London: Centre for Longitudinal Studies , I n s t i t u t e o f E d u c a t i o n . 2 0 0 2 . A v a i l a b l e f r o m : http://www.cls.ioe.ac.uk/studies.asp?section=0001000200020005
52. UK Data Service Discover » 1970 British Cohort Study: Twenty- N i n e – Y e a r F o l l o w – U p , 1 9 9 9 – 2 0 0 0 [ I n t e r n e t ] . http://dx.doi.org/10.5255/UKDA-SN-5558-2
53. Rutter M, Tizard J, Whitmore K. Education, health and behaviour. London: Longman; 1970.
54. Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, et al. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;
Sep 8;132(10):965–86.
55. Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012; May;30(5):842–51.
56. Li Z, Li Y, Chen L, Chen P, Hu Y. Prevalence of Depression in Patients With Hypertension: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015; Aug;94(31):e1317.
57. Harpole LH, Williams JW, Olsen MK, Stechuchak KM, Oddone E, Callahan CM, et al. Improving depression outcomes in older adults with comorbid medical illness. Gen Hosp Psychiatry. 2004; Jan;27(1):4–12.
58. Matheson FI, Smith KLW, Fazli GS, Moineddin R, Dunn JR, Glazier RH. Physical health and gender as risk factors for usage of services for m e n t a l i l l n e s s . J E p i d e m i o l C o m m u n i t y H e a l t h . 2 0 1 4 ; Oct;68(10):971–8.
***




