METABOLIC ASSESSMENT AND QUALITY OF LIFE IN A SAMPLE OF PATIENTS IN TREATMENT WITH OLANZAPINE DEPOT
Abstract
BACKGROUND: Non adherence to medication is a major risk factor contributing to relapse and hospitalization among patients with schizophrenia. Treatment with antipsychotics in depot formulations is recognized as safe and effective for improving medication adherence.(1) OBJECTIVES: Metabolic and quality of life assessment of patients treated with olanzapine depot METHODS: We studied 24 patients in treatment with olanzapine depot, monitored between 2011-2017. For metabolic assessment we used data from patients' clinical recordings (variation in Blood Pressure, glycaemia , cholesterol, triglycerides, BMI) and for quality of life assessment SF-36.questionnaire and data regarding socio-professional, familial reinsertion, number of relapses and hospitalisations obtained from clinical interview. RESULTS: The studied sample comprised 16 men and 8 women, diagnosed with schizophrenia, most of them being previously treated with oral olanzapine. Most of the patients were under treatment with olanzapine depot 300mg i.m.,two shots per month (at 2 weeks distance). Two patients lost weight, the majority of them had no significant variation in BMI, one patient with increase of 8,4units in BMI, two patients with stable BMI. 7 of 12 patients had increased cholesterol levels, the rest of biological parameters being normal. Two patients had post-injectional somnolence. 13 patients had no relapses, 3 patients were noncompliant, 3 had relapses, 3 were lost from monitoring and 2 were changed on orally administered olanzapine. 4 patients had total score above 50 at the mental component of SF-36 and 4of them had total scoreabove 50 at the physical component of SF-36. CONCLUSION: In the studied sample, most of the patients had a favourable evolution regarding clinical aspects, familial and socio-professional reinsertion and metabolic outcomes, which confirms the fact that olanzapine depot is a safe treatment associated with low incidence of relapses. Further studies are needed on large samples, in order to evaluate quality of life in patients treated with olanzapine depot.
BACKGROUND:
Non adherence to medication is a major risk factor contributing to relapse and hospitalisation among patients with schizophrenia(1). Treatment with antipsychotics in depot formulations is recognized as safe and effective for improving medication adherence and it appears to benefit n o n c o m p l i a n t p a t i e n t s w i t h s c h i z o p h r e n i a ( 1 ) . Hospitalization is a costing and stressful event associated with relapse during treatment of patients with schizophrenia (2). Olanzapine depot was associated with a significant lower incidence of hospitalizations and a shorter duration of hospitalization compared to olanzapine administered orally in therapeutic dose (2).
METHODS:
We studied 24 patients in treatment with olanzapine depot, monitored between 2011-2017. At every visit patients were monitored for 3 hours in the hospital(clinical state and vital signs). For metabolic assessment we used data from patients’ clinical recordings (variation in Blood Pressure, glycaemia, cholesterol, triglycerides, BMI) and for quality of life assessment SF-36 questionnaire which we applied to 6 of the 13 patients who are still monitored and data regarding socio-professional, familial reinsertion, number of relapses and hospitalisations obtained from clinical interview.
RESULTS:
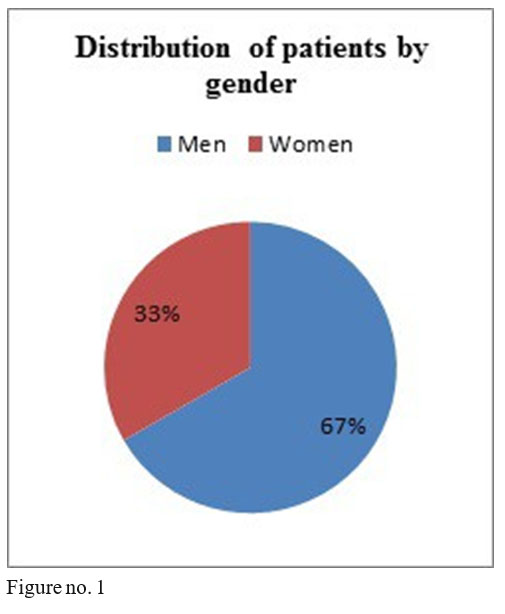
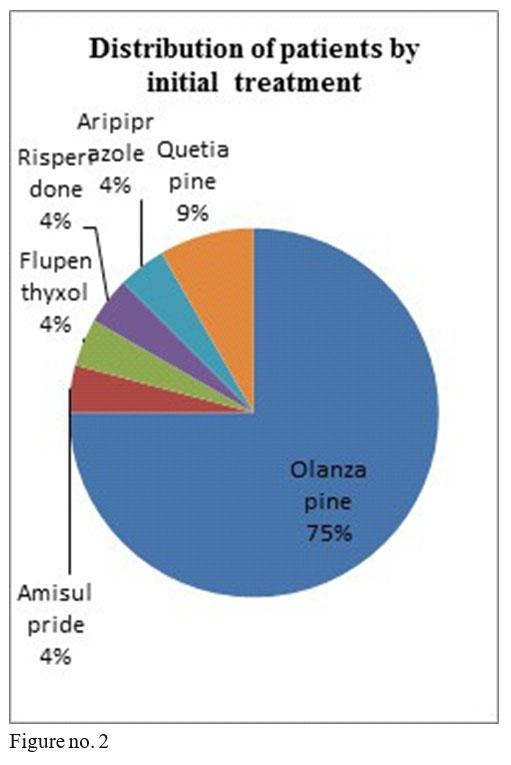
The studied sample comprised 16 men and 8 women, minimum age 28, maximum age 57 at the beginning of treatment, diagnosed with schizophrenia, 18 patients in anterior treatment with oral olanzapine, 1 with flupenthyxol, 1 with aripiprazole, 1 with amisulpride, 2 with quetiapine and 1 with risperidone.
Olanzapine depot dosage used were the following: 8 patients on 405 mg/month i.m., 15 patients with 300mg /2 weeks i.m., one patient with 210mg/2 weeks.
Biological parameters monitored:
BMI-2 patients lost weight, most of them without significant variation in BMI, one patient with significant variation (an increase of BMI of 8.4 units), 2 patients with stable weight. Glycaemia- values were in normal limits for all monitored patients.
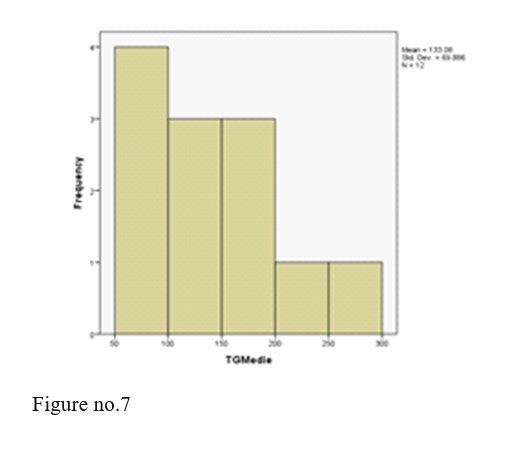
Triglycerides- all values within normal limits.
Cholesterol- 7 out of 12 patients had values above the normal limit.
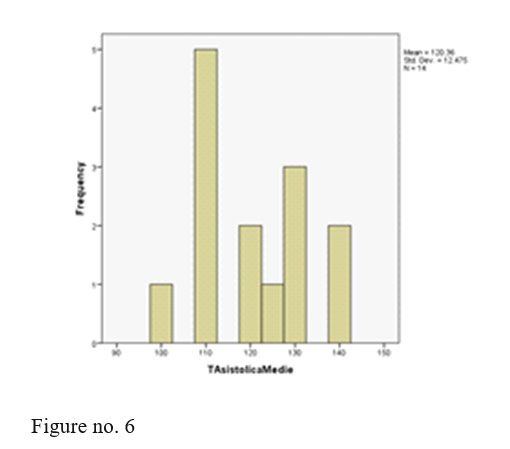
BP and pulse- values were in normal limits for all monitored patients.
TGO and TGP were normal in majority of patients.
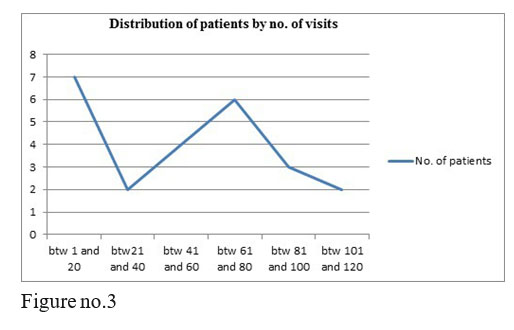
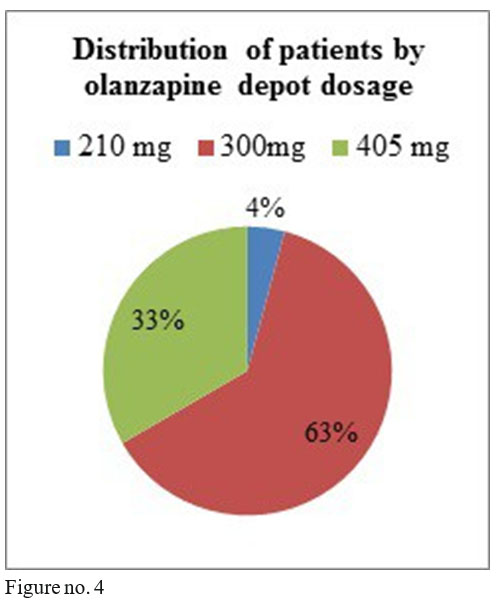
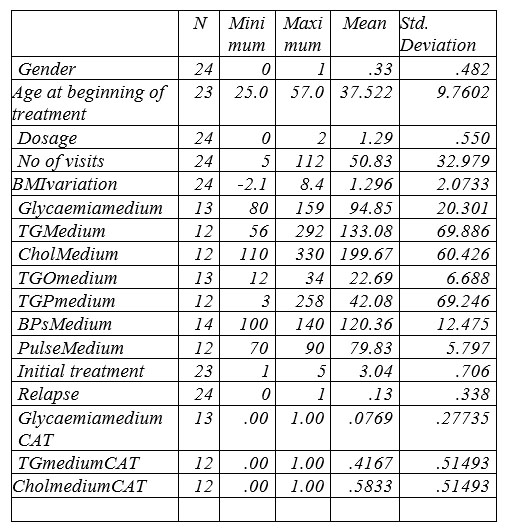
Table no.1 Descriptive Statistics
Conventions: 0=man Dosage: 0=210 1=300 4=405 For a simple analysis we kept only systolic BP values.
We registered 2 adverse events, two patients with post injection somnolence. The two patients were administered i.v. fluids and were monitored until next day in the hospital, their outcome being favourable and continued treatment with olanzapine depot with good evolution at the present time.
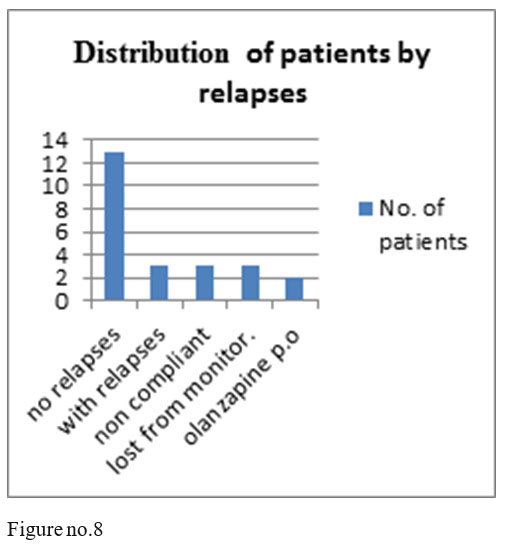
13 patients of the 24 monitored ones had no relapses, 3 patients had relapses after 5 yers of treatment with olanzapine depot, 3 patients were noncompliant after 5 years of treatment, 3 were lost from monitoring and 2 were changed on orally administered olanzapine, on request
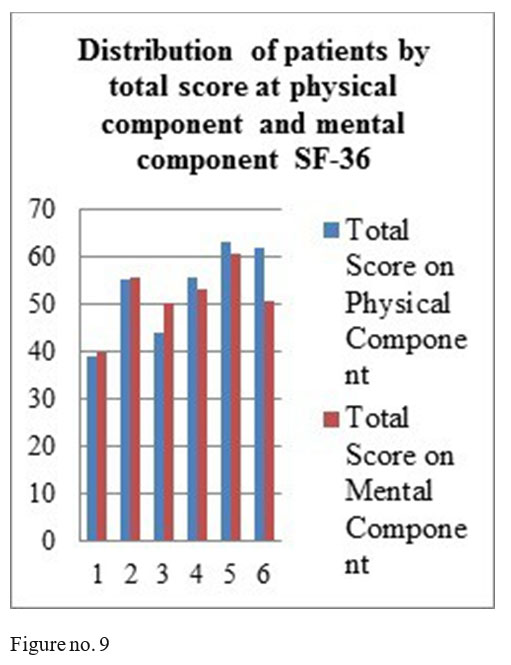
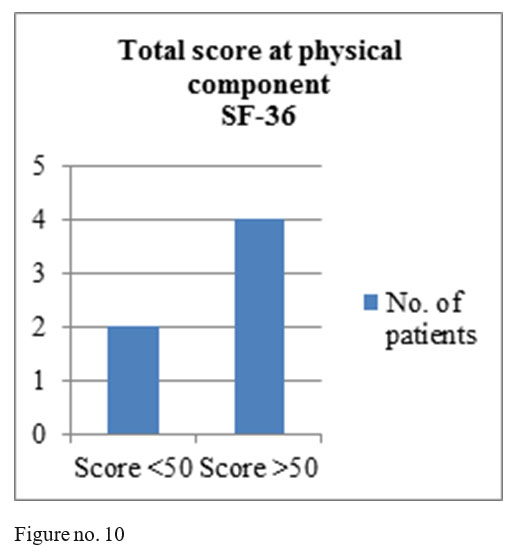
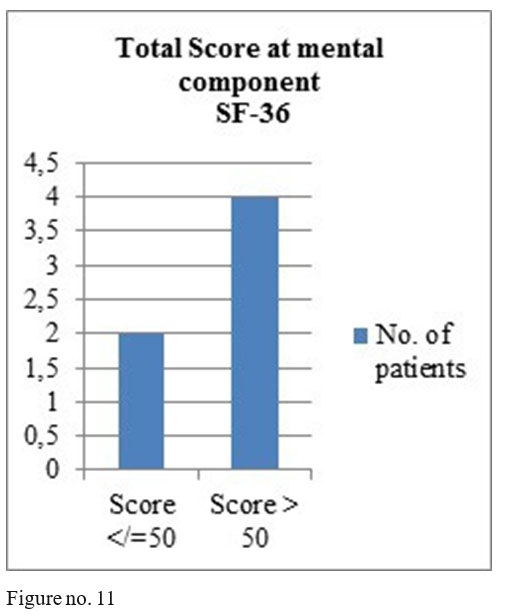
SF-36 questionnaire results: mental component- 4 patients obtained total score above 50, one patient a total score of 50 and one patient a score under 50; physical component- 4 patients with the total score above medium and 2 with a score under the medium.
3 of our studied patients are employed, most of the monitored patients come alone to treatment, after only 2-3 years of treatment they became independent, all of the patients respect the visits’ schedule and they stay 3 hours in the hospital, being monitored at every visit.
CONCLUSION:
Schizophrenia is a chronic disease in which relapses are frequent during the life of the patients (2,3). During the relapse the patient might be admitted to hospital for acute treatment and control of psychotic symptoms, fact that can lead to added costs and delays recovery (4,5), reducing quality of life in these patients(6). This is why, it must be found a treatment that contributes to increase in adherence, lowering of the relapses and the costs and improvement in quality of life of the patients.
In the studied sample, most of the patients had a favourable outcome regarding clinical aspects, familial and socio-professional reinsertion and metabolic evolution, which confirms the fact that olanzapine depot is a safe treatment associated with low incidence of relapses which implies a low number of hospitalisations.
Further studies are needed, on larger samples, in order to evaluate quality of life in patients treated with olanzapine depot.
REFERENCES:
1.Ascher-Svanum H, Montgomery W, McDonnell D, Coleman K, Feldman P. Treatment-completion rates with olanzapine long-acting injection versus risperidone long-acting injection in a 12-month, open- label treatment of schizophrenia: indirect, exploratory comparison. International Journal of General Medicine 2012; 5: 391–398.
2.Ascher-Svanum H et al. Predictors of psychiatric hospitalization during 6 months of maintenance treatment with olanzapine long-acting injection: post hoc analysis of a randomized, double-blind study. BMC Psychiatry 2013; 13:224. http://www.biomedcentral.com/1471- 244X/13/224
3.Citrome L. New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Rev of Neurother 2013; 13(7):767-783, DOI: 10.1586/14737175.2013.811984
4.Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999, 56:241–247.
5.Lieberman JA, Koreen AR, Chakos M, Sheitman B, Woerner M, Alvir JM, Bilder R: Factors influencing treatment response and outcome of first episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry 1996; 57(Suppl 9):5–9.
6. Almond S, Knapp M, Francois C, Toumi M, Brugha T: Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry 2004; 184:346–351.
Figure no. 11
***




