PREVENTION AND TREATMENT OF POSTOPERATIVE DELIRIUM IN THE ELDERLY, NON-CRITICALLY ILL PEOPLE: A LITERATURE REVIEW
Abstract
Deliriumul este o patologie neuropsihiatrica severa subdiagnosticata cu implicatii pe termen scurt si lung in ceea ce priveste riscul de moarte, calitatea vietii si povara economica. Exista destul de multe date in literatura in ceea ce priveste deliriumului in unitatile de terapie intesiva sau de medicina interna, dar mult mai putine date in cele chirurgicale. Scopul articolului nostru a fost de a sumariza datele relevante si actuale cu privire la preventia si tratamentul deliriumului postoperator. Pentru aceasta am cautat bazele de date PubMed si Cochrane dupa urmatoarele cuvinte: “delirium”, “postoperator”, “preventie”, “tratament”, “chirurgie”, “varstinici”. In momentul de fata nu exista markeri validati in a determina persoanele la risc de a suferi de delirium postoperator. De asemenea, nu exista niciun ghid care sa normeze preventia sau tratamentul deliriumului. Chiar si asa, terapiile multicomponente nonfarmacologice care includ mobilizarea precoce, orientarea vizuala si spatiala, reglarea somnului etc. par sa aibe rezultate pozitive in ceea ce priveste preventia deliriumului, dar nu si tratamentul acestuia. Pana in momentul de fata nu exista un consens in ceea ce priveste preventia sau terapia farmacologica. Pe aceasta tema, literatura este divizata din cauza numarului mic de studii randomizat controlate ce folosesc terminologii diferite, au heterogenitate mare si o raportare deficitara a efectelor adverse. Cu toate acestea exista molecule precum suvorexantul sau dexmedetomidina care par sa fie eficiente in preventia deliriumului postoperator, dar care sunt totusi departe de validarea clinica. In concluzie, aceasta tema importanta si de multe ori neglijata a deliriumului necesita aprofundarea prin studii omogene si randomizat controlate la toate nivelurile – stiinta fundamentala, stiinta translationala si teste clinice.
INTRODUCTION
Delirium is a common neuropsychiatric affliction, frequently underdiagnosed and undertreated, with severe long and short-term outcomes, increased perioperative risk of death and a marked economic burden ranging up to and exceeding 152 billion $ per year in the USA, rivaling the health care costs of Diabetes Mellitus (1-5). Delirium is represented by the sudden onset of cognitive impairment and altered consciousness. The syndrome incorporates fluctuations of consciousness over the course of a day, decreased focus and evidence that the cause is organic, in direct relationship with the underlying condition. Although delirium has been studied with mixed results in a plethora of settings (medical, surgical and ICU), the prevention or treatment of the elderly non- critically ill surgical patient is one of the least studied subjects delirium-wise. The aim of our review is to converge all the relevant data on this topic in this article. As such, in a surgical setting, the incidence varies from 11 to 51%, with Orthopedic Surgery and Cardiac Surgery leading in the top, the target population being the elderly, most medically complex patients (2). In general, the incidence is higher in the acute procedures compared to the elective ones, such as orthopedic repair fractures (21.7%) vs elective orthopedic surgeries (12.1%) and is also dependent on the complexity of the surgical act – valve replacements having higher rates of delirium than the less difficult bypass surgery (6, 7). That is not to say that minor surgeries are without delirium risks. A study aiming to explore the incidence of delirium following cataract surgery found that 4.4% of their subjects manifested immediate postoperative delirium and that older age and more frequent use of benzodiazepine medications were risk factors [8]. Another study found that the incidence of postoperative delirium in a lot of 358 people undergoing elective Trans Urethral Resection of the Prostate (TURP) was 7.8%, with old age and pain intensity after surgery being found as risk factors (9). The implications of these high rates are far reaching and span over both the medical and economical aspects of patient care. It has been shown than persons who develop delirium are at a greater risk of death (both in the hospital and after discharge), long-term cognitive decline, lengthy hospitalizations, reduced physical function for more than 30 days after the procedure, reduced quality of life and need of institutionalization (2)(4)(10). Thus, the interest in this area is spurred, as studies have shown delirium can be prevented in as much as 30-40% of the cases, it’s severity reduced, as well as the total number of days with delirium minimized (11, 12, 13). Multiple theories have been proposed on the pathogenesis of delirium and the seven most popular ones are: the neuro-inflammation, the neuronal aging, the oxidative stress, the neurotransmitter, the neuroendocrine, the diurnal dysregulation or melatonin dysregulation and the network disconnectivity hypotheseses (14). The neuro-inflammatory theory postulates that the acute peripheral inflammation induces the activation of the parenchymal cells and the expression of pro-inflammatory cytokines at the CNS level which in turn create an imbalance in the neural and synaptic enviroment, all of this leading to the neurobehavioural symptoms of delirium. The neuronal aging theory stands on the concept of homeostenosis which states that even though an elder can be functional and healthy into old age, he is more prone to illness due to a lack of neuronal physiological reserve. The oxidative stress theory stands on the concept that hypoperfusion induces oxidative damage in cells with the generation of reactive oxygen and nitrogen species. The neurotransmitter hypothesis states that delirium is linked to a low central cholinergic transmission mixed with an increased dopaminergic activity, both of which have an effect on the GABA-ergic and glutamatergic pathways. The neuroendocrine theory suggests that neurons enter a vulnerable state due to abnormally high glucocorticoids levels which makes them unable to survive after acute metabolic insults. The diurnal dysregulation and melatonin dysregulation hypothesis both rest on the the fact that the impairment of the normal 24-hours sleep cycle and of the physiological sleep stages lead to delirium through an imbalance in natural-killer cells, reduced IL-2 production and other inflammatory mediated mechanisms. Finally, the network disconnectivity theory states that different delirium subtypes correspond to different neurotransmitter- specific alterations with the main culprits being the cholinergic and GABA-ergic systems. It is unlikely that delirium is the sole result of just one of these theories and more probable that the above mechanisms interact with one another. As such, delirium occurs as the interplay of a patient’s predisposing factors and the surgical and anesthesical stress sustained in the perioperative period. The single most common risk factor is advanced age (>65 years old). Other than this, predisposing factors include: preexisting neuropsychiatric conditions, habitual use of psychotropic medications, poor physical status, diabetes mellitus, atrial fibrillation, atherosclerosis and tobacco use. Precipitating factors include surgery duration and complexity, blood lose or transfusion, depth of anesthesia, postoperative complications such as infections, pain, sleep disruption or use of physical restraints (15). Different surgical fields have different weighing risk factors for delirium. For example, in vascular surgery, elderly patients having renal failure, previous strokes or male sex are strongly associated with postoperative delirium whereas in deep brain stimulation surgery for Parkinson disease, white matter atrophy in the temporal stem is a factor predictive of postoperative delirium (16) (17). Also, in elderly patients (>65 years old) undergoing non-cardiac surgery and especially in colorectal cancer operations, hypoalbuminemia was found to be a strong risk factor associated with postoperative delirium (18) (19). The problem is that risk factors alone cannot predict the onset of delirium. To remedy this, new promising studies are using the pathophysiological theories of this illness to find humoral or imagistic vulnerability markers that can estimate the probability of postoperative delirium or its severity. Such a study (20) revealed that the neuroticism score, the amplitude of low-frequency fluctuations in the dorsolateral prefrontal cortex and the gray matter density in the caudate/suprachiasmatic nucleus are predisposing factors. A postoperative delirium prediction model including this variables showed a correct classification rate of 86%. Other studies seem to indicate that the neopterin levels in the cerebrospinal fluid can be used as a predictor of both the onset of postoperative delirium and its poor outcomes (21) (22). The CRP could also be used as a biomarker for predicting delirium in non- cardiac surgery, but its low specificity for this pathology could be a hindrance (23). Also, the cerebrospinal fluid beta-amyloid 1-42 was proposed as a possible prediction marker, but studies seem to talk at cross purposes on the matter of its usefulness (24) (25). It is worth mentioning, though, that there are far too few studies on the CSF B- amyloid 1-42 as a marker for delirium and the subject warrants further research to come to a definitive answer. On another note, reduced MMSE scores on the delayed recall and working memory domains appear to be predictors of delirium (26). It is important to bear in mind that the studies regarding the prediction of the onset of postoperative delirium are too few and far between and is a field worth investigating deeper.
Clinically, delirium presents itself in 3 forms: hyperactive, hypoactive and mixed. The hyperactive form is the most recognisable one by the medical staff as it’s presented with hypervigilance and psychomotor agitation. Conversely, hypoactive delirium mainly goes unnoticed because the lethargy and slowing might not be readily evident, but also carries a worse prognosis than the hyperactive one and it occurs more often. The mixed form is a combination between the aforementioned two.
DIAGNOSIS
Postoperative delirium is mainly a clinical diagnosis and is prone to the subjectivity of the observer. Studies have shown that even with a standardized assessment tool, its sensibility and specificity can decrease if used by an untrained professional (2). Henceforth, numerous clinical tools have been developed to satisfy this need. By far, the most used and studied tool is the Confusion Assessment Method (CAM) and its variants like CAM-ICU. The CAM tool assesses the presence, severity, and fluctuation of 9 delirium features: acute onset, inattention, disorganized thinking, altered level of consciousness, disorientation, m e m o r y i m p a i r m e n t , p e r c e p t u a l d i s t u r b a n c e s , psychomotor agitation or retardation, and altered sleep- wake cycle. The presence of delirium requires the presence of acute onset and inattention plus one of either disorganized thinking or altered level of consciousness. A
2010 systematic review validated CAM as having the best available evidence for its use as a bedside delirium tool (27). More than this, CAM only takes around 5 minutes to administer. Interestingly enough, this same study found that the MMSE scale was the least useful at identifying patients with delirium. There is a plethora of other tools for identifying delirium adapted for various situations such as the Short Orientation Memory Concentration Test (28) which is best suited in short outpatient encounters, or the Nursing Delirium Screening Scale (29) which is best used in an inpatient setting with continuous surveillance. Newer clinical tools that need further testing include the Delirium Observational Screening Scale (30), the 4AT (31) and the Months Of The Year Backwards (32). Still, postoperative delirium remains under-recognized in the surgical setting at all levels – doctors, nurses, family – and its symptoms are poorly documented in the medical notes, especially when superimposed on pre-existing dementia (33). This could be due to lack of information on the subject, poor assessment tools and poor nomenclature. Thus, further insight and clarifications on the matter is much needed.
NON-PHARMACOLOGICAL PREVENTION AND TREATMENT
For the sake of clarity, we have included in the non- pharmacological category all the interventions not related to psychoactive medication. As such, blood transfusions, saline infusions and physical preconditioning will all be described here.
Many of the risk factors for delirium are amenable by simple non-pharmacological measures.
While some risk factors are immutable (age, preexisting cognitive impairment, multiple comorbidities), others are not (sleep imbalance, immobility, visual and hearing impairment, dehydration, social isolation, physical restraint use, psychotropic polypharmacology, pain, hypoxia, Foley catheterization and more) (10). However, things are more complicated than they look at a first glance. Even though a lot of the risk factors are common between medical and surgical delirium patients, there are still some that are particular to the latter (higher pain, immobility, etc.). Thus, prevention and treatment of delirium in a surgical setting might require a different approach and could be met with a different set of challenges than in a medical setting.
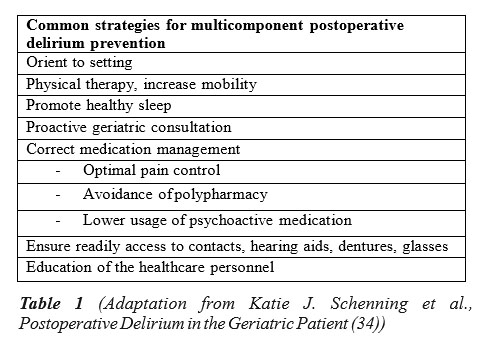
Some of these risk factors can be easily targeted non- pharmacological in a multidisciplinary manner or with proactive geriatric consultations. The hallmark study that addressed in a nonpharmacological manner 6 of the above risk factors – cognitive impairment, sleep deprivation, immobility, visual impairment, hearing impairment, dehydration – was developed by Inouye et all (12) and was met with resounding success in both decreasing the incidence and the number of delirium episodes. Later, this whole strategy has become known as the Hospital Elder Life Program (HELP) and has been successfully implemented in multiple countries across the world. The initial study was focused on medical wards patients with delirium and thus some of the components of HELP could not be accurately reproduced in a surgical setting. To address this, numerous adaptations of HELP have been developed, all sharing the basic strategies summarized in Table 1 (34) and showing similar efficacy in preventing delirium in oral cancer surgery (35), hip surgery (36) or abdominal surgery (37).
All in all, a recent Cochrane Review revealed that the incidence of delirium had a risk ratio of 0.69 (95% confidence interval, 0.59-0.81) when comparing multicomponent non-pharmacologic intervention to normal hospital care (38). To corroborate this, a systematic review on the effectiveness of multicomponent interventions on the incidence of delirium in hospitalized older patients with hip fracture found that the incidence of postoperative delirium was lower in the intervention group and also that the cognitive decline was higher in the control group compared to the intervention one (39). On the other hand, the same systematic review did not shed light on the duration or the severity of the delirium. In regards with duration, the studies are not completely relevant, 3 studies could not be pooled, 4 studies reported significantly lower delirium duration in the intervention group and 2 studies did not find it statistically significant.
Interestingly enough and warranting further research, this systematic review found only 3 studies on the functional and mobility status and only one of them found that a significantly higher number of participants were walking independently with walking aids on discharge in the intervention group compared to the control one. Another systematic review found that the HELP program is effective at reducing delirium incidence and rate of fall, with a trend towards reducing length of stay and preventing institutionalization (40).
A great deal of studies (41) (42) have come to the conclusion that many of the multicomponent non- pharmacological interventions do not require trained professionals to put them in practice, but rather similar results can be achieved with family members, caregivers or volunteers. One drawback is that these studies were focused on the medical wards patients, whereas there is currently a scant body of evidence on the surgical patients. One of the few randomized studies found evaluated the effects of involving family members in the management strategies for delirium in a post cardiac surgery setting (43). The study showed promising results, with the intervention group presenting better psycho-functional recovery scores when compared to the control. However, its limitations were important – low number of participants (14 in usual care group and 16 in the intervention one) and most of all, the intervention happened after the surgery so there was no pre-operative preventing component in it. We believe this specific topic needs further evidence-based attention in a multitude of surgical specialties and we think that the simple involvement of families or volunteers in preventing delirium (for eg. reorientation, restoration of sensory perception, oral hydration etc.) in a surgical setting could yield similar results to the medical one while also improving the cost-effectives of the whole process.
In addition to the multicomponent strategies presented above, numerous other non-pharmacological methods have been tested in trying to prevent postoperative delirium. Blood transfusions used to correct anemia have mixed evidence when it comes to postoperative delirium prevention (44) (45). Hypertonic saline solution, widely used in the resuscitation of the traumatic hemorrhagic shock, has been proven to have beneficial effects on the immune cell function and the subsequent inflammation. Thus, a novel approach uses hypertonic saline solutions to prevent delirium with promising results (46) (47) (48). On the other hand, preoperative exercise capacity is an independent factor in the development of postoperative delirium in elective cardiac surgery (49). It remains to be seen if physically preconditioning a patient at risk before an elective surgery can reduce the incidence of delirium. Bright light therapy and music therapy, although having strong recommendations against their use in an ICU setting, might prove effective in a surgical setting where the patients have a different biological profile (50). Further research should investigate the effect of multicomponent nonpharmacological interventions on the biological markers associated with delirium (IL1, Il6, TNFalpha, cortisol, CRP, etc.). Also, it is important to keep in mind that once postoperative delirium develops, the current evidence does not support the efficacy of multicomponent non-pharmacological interventions to treat it (51).
P H A R M A C O L O G I C A L P R E V E N T I O N A N D TREATMENT
Multiple pharmacological therapies have been researched regarding their efficacy in preventing and / or treating delirium. Summarized below are the main classes most investigated.
A)Typical antipsychotics
Of the typical antipsychotics, probably the most studied is Haloperidol. Although there is much data regarding the prevention and treatment of postoperative delirium in the ICU patients, that is not the case with the ordinary postoperative delirium patient on the surgical wards. Moreover, results regarding the prevention and treatment with haloperidol of the ICU patients (52) (53) cannot be extrapolated to the normal postoperative delirium patients because the former have a poorer biological status and a different pharmacological safety profile. Taking this into consideration, data on the run-of-the-mill postoperative delirum patients is divisive. One study came to the conclusion that prophylaxis with haloperidol can significantly reduce the incidence of delirium in the elderly (54), while Fukata et al. states that, after conducting a randomized, open-label prospective trial on the effects of haloperidol prophylaxis on postoperative delirium in the elderly, there were no significant differences between the intervention and the control groups (55). In all of the above studies, the prophylaxy was made with low dose haloperidol, but the definition of that low dose and the method of administration (eg. 1 time 2.5 mg bolus, 3 times per day x 0.5 mg, continuous iv 0.1 mg/g x 12h) differs vastly which can be a confounding factor in pooling the studies.
On the other hand, regardless of its ability to prevent delirium, it seems haloperidol might be useful in reducing the duration, severity and length of stay according to the above meta-analysis (54), but even this is still a matter of debate. As such, a Cochrane Database Systematic Review updated in 2018 found that typical antipsychotics did not reduce delirium severity, resolve symptoms or alter mortality (56). This dichotomy only reinforces the need for high quality studies and for further research in this matter. Once postoperative delirium develops in non-ICU patients, treating it with haloperidol is met with a paucity of evidence. The same Cochrane Review found that typical antipsychotics did not resolve delirium symptoms compared to nonantipsychotic drug regimens, but also marked it as a very low-quality evidence. This seems to be corroborated by another systematic review, also taking into consideration the same very-low quality evidence (57). On the opposite side stands Kishi et al. whose meta- analysis came to the conclusion that antipsychotics were significantly superior to placebo in response rate in the non-ICU setting (58).
On the positive side, in most of the above studies there were no significant side-effects present in the non-ICU delirious patients (including similar QTc in both the intervention and the control groups) at the administration of the low-dose haloperidol prophylaxy. To top it, Schrijver EJ et al. found that low dose oral haloperidol does not seem to prolong the QTc interval in the elderly acutely hospitalized (59). This warrants further research into this matter, as this new finding in the safety profile of haloperidol in the treatment of non-ICU patients with delirium could be a pivotal shift into issuing guidelines. At the moment, taking into consideration the possible side- effects of haloperidol (cardiac arrhythmias, QTc prolongation, pancytopenia, extrapyramidal reactions etc.), the current guidelines state that haloperidol should not be used for either prophylaxis or treatment of postoperative delirium and its use in a delirium context should be reserved only for the patients with severe psychosis and agitation, that present a risk to themselves or others (60).
B)Atypical antipsychotics
-Olanzapine
Olanzapine is a second generation antipsychotic with antagonistic effects on the serotonin and dopamine postsynaptic receptors. Although there are not nearly as many studies conducted on the topic of postoperative delirium on Olanzapine as there are on Haloperidol, there is still enough data to form a modicum of opinion. As is the case with Haloperidol and, for all intents and purposes with all antipsychotics, there are not so many studies investigating the non-ICU surgical setting as there are on the topic of ICU patients. Taking this into consideration, a study comparing Olanzapine vs Placebo for the prevention of postoperative delirium in elective hip- replacement patients found that the administration of 10 mg of oral Olanzapine perioperatively was significantly associated with a lower incidence of delirium. However, delirium lasted longer and was more severe in the olanzapine group, and patients treated with olanzapine had lower albumin and calcium levels, increased prolactin levels and abnormal lipid and glucose metabolism (61). Another inquiry on the same database of the aforementioned study found that patients who received Olanzapine had an 83% risk of developing delirium if they received more than 42.5 mg equivalents of intra-operative morphine, had >= 74 years old and had a mean arterial pressure < 90 mmHg at the presurgical screening visit (62). With more studies and data like this, lines could be drawn to isolate a target group on which Olanzapine attains optimal results as a prophylactic agent.
When it comes to treating postoperative delirium, in their meta-analysis, Kishi et.all found that Olanzapine din not outperform Haloperidol when it came to alleviating the delirium, but the former was superior to the latter regarding time to response (TTR) and the incidence of dystonia. Olanzapine was also associated with a higher incidence of dry mouth compared to placebo (58).
-Quetiapine, Risperidone and Ziprasidone
Data on these three antipsychotics is scarcer even than data on the former two – Haloperidol and Olanzapine. Quetiapine seems to have a positive outcome on time to response, response rate and delirium severity scale scores at study endpoint when compared both with placebo and haloperidol (58). Also, a study on the prevention and treatment of postoperative delirium in the elderly following elective spinal surgery came to the conclusion that adding quetiapine to haloperidol results in a delirium resolution an average of 3.5 days faster that haloperidol alone (P=0.001) and decreases agitation and length-of- stay in hospital (P=0.02 and P=0.05 respectively) (63). Risperidone, in turn, is sometimes preferred because it is less sedating. One study found that one dose of 1 mg of risperidone decreased the incidence of postoperative delirium after cardiac surgery with cardiopulmonary bypass by 20% (64). Building on this idea, Grover et al. states that risperidone is as efficacious as haloperidol in treating delirium, but as a downside he did not study its effects on preventing delirium (65). Ziprasidone is by far the least studied of the above antipsychotics when it comes to delirium. No studies were found on the prevention and/or treatment of postoperative delirium on non-critically ill patients and one of the few studies on the ICU patients found no significant difference in the outcomes of treating delirium with ziprasidone, haloperidol and placebo (66). It is important to note that most of the research on the topic at hand involving the above 3 atypical antipsychotics is offset by small sample populations and the road to a more definitive answer is long and fraught with scientific uncertainty.
-Other atypical antipsychotics
When it comes to the quality of evidence on atypical antipsychotics including Amisulpride and Aripiprazole, it is important to note that Rivière J et al. mentioned in their 2018 systematic review that there are no randomized controlled studies to support their efficacy and tolerability delirium-wise (67). These two atypical antipsychotics are by far the least studied in a surgical setting. Amisulpride has been shown to provide good results in the treatment of postsurgical delirium, but no data is available for its use in prevention and, more so, the results are limited by a small population sample (68). On the other hand, to the best of our knowledge, aripiprazole has not been tested in a controlled manner in a surgical delirium setting. If we were to extrapolate its results in a medical / palliative care setting, it could be an effective treatment option in a surgical environment (69).
Trying to reach a conclusion on the use of antipsychotics in preventing or treating delirium at this moment in time is impossible. Although the current body of evidence indicates that the use of antipsychotics on non-critically ill elderly patients yields better results than on the critically ill, both regarding the outcomes and the safety profile, with second generation antipsychotics being at least as efficacious as haloperidol and more safe, the matter still stands under a shadow of a doubt. As long as there are Cochrane reviews that state the data on this topic is of poor quality and the safety profile could not be properly assessed because the adverse effects were poorly or rarely reported (56), the use of antipsychotics in postoperative delirium should be done sagaciously using a “start low and then go slow” attitude.
C)Sleep cycle altering medication
The diurnal dysregulation or melatonin dysregulation is one of the current leading hypothesis when it comes to the pathogenesis of delirium. Sleep deprivation has long been linked to the development of delirium. Melatonin has been demonstrated to play a major role with its chronobiotic, s l e e p – w a k e c y c l e r e g u l a t i o n , a n t i o x i d a n t a n d antiapoptotic, anti-inflammatory, anti-nociceptive and analgesic effects and its ability to prevent the hyperphosphorylation of the tau protein (14). Knowing all this, it was only natural to try this avenue in preventing or treating delirium. The results, unfortunately, have been met with mixed results. A recent meta-analysis conducted by Chen et al. included 189 medical patients and 480 surgical patients from 4 randomized control studies and came to the conclusion that no differences were found in jthe incidence of delirium between the two groups (melatonin intervention vs control) in the elderly patients that were presented to surgical wards (70). What is important and interesting to note is that regarding the medical patients there was a statistically significant reduction of 75% in the incidence of delirium. Also, one of the studies on surgical patients included in the analysis reported a decrease of the long-lasting episodes of delirium (>2 days) in the melatonin group. This conclusion should not be taken at face value, as the meta- analysis was marred by a high heterogeneity and lack of uniformity in the diagnostic criteria of delirium in the surgical studies subgroup analysis. The authors state that the small sample might not be consistent with the clinical practice and advise caution before applying these results in clinical practice as there is a dire need of more trials to determine the exact effect of melatonin on surgical patients with delirium. Interestingly enough, another study conducted after Chen’s meta-analysis came to the conclusion that administration of melatonin significantly decreases the incidence of delirium after cardiac surgery, so the game is still on (71).
Ramelteon is a melatonin agonist and is regarded as having a sixfold and threefold higher affinity for melatonin receptors 1 and 2 (MT1 and MT2) respectively, when compared to melatonin in vitro (70). Despite its potency, it is less studied on preventing surgical delirium than melatonin. Two recent studies indicate that ramelteon is associated with a significant reduction in postoperative delirium incidence in both lung cancer surgery and pharyngolaryngectomy with esophagectomy surgery (72) (73). More evidence is needed though to draw a conclusion.
A new promising molecule is Suvorexant whose mechanism of action is by antagonizing the orexin receptors, orexin being one of the neuropeptides responsible for the state of wakefulness, thus promoting sleep. One recent study indicates that the Suvorexant intervention group reported significantly lower delirium incidence after coronary artery bypass grafting (CABG) surgery (74). Another study tried to see the effects of ramelteon and suvorexant combined on the incidence of postoperative delirium, but the results were inconclusive (73).
When it comes to delirium treatment with melatonin or ramelteon, data is of low quality and comprised mostly of case reports or retrospective cohort studies. There are more articles on ramelteon than melatonin as a treatment for delirium and these seem to indicate a reduction both in the severity and the duration of delirium (75). This lead looks promising but is in dire need of more statistically- oriented evidence.
Other aspects worth studying in further trials are the effects of melatonin / ramelteon in preventing the different subtypes of delirium (hypoactive, hyperactive and mixed) and the effects of different doses of melatonin/ramelteon in preventing delirium. When it comes to the first aspect, it has been shown that urinary excreted 6-SMT (6- sulfatoxymelatonin) is higher in hypoactive delirium and lower in hyperactive delirium (76) so it could well be that the different subtypes of delirium might respond differently to the administration of melatonin. Regarding the second aspect, a higher dose of melatonin might inhibit the endogenous melatonin secretion through a negative feedback mechanism (77).
D)Cholinesterase Inhibitors
Low Acetylcholine has been linked to delirium (14). By inhibiting its degradation and consecutively improving the amount found in the synaptic space, it has been theorised that cholinesterase inhibitors can prevent delirium and slow the progression of dementia. Rivastigmine and Donepezil are two of the most studied drugs of this class.
Data on Rivastigmine is conflicting. When it comes to preventing postoperative delirium, one study showed no significant difference in reducing its incidence (rivastigmine intervention vs control) in electiv cardiac surgery (78), while another study conducted on a hip fracture surgery population with preexisting cognitive impairment stated that rivastigmine reduced both the incidence and the mean severity of delirium (79).
The scarce data on Donepezil does not seem to support this drug at the moment. One study on an elderly hip fracture group found no significant reduction on postoperative delirium incidence and the intervention group experienced significantly more side effects compared to the control (80). Another study conducted on elective hip replacement patients also found no statistically significant reduction in delirium incidence, but the authors stated that there had been a consistent trend suggesting a possible benefit (81). It is important to note that both studies had small sample populations and that the acute setting of the first study vs the chronic one of the second can seriously impair drawing a conclusion on the matter at hand.
There is currently insufficient evidence derived from controlled trials suggesting that either Rivastigmine or Donepezil could be effective in treating postoperative delirium (82) (83).
E)Anesthetics
Cerebral hypoperfusion in the frontal, temporal and occipital cortex as well as in the thalamus and basal ganglia has been associated with postoperative delirium (84). Low cerebral blood flow is present in anesthesia induced by both intravenous propofol and volatile general anesthetics like Sevoflurane, Desflurane and Isoflurane. A 2018 Cochrane Database Systematic Review could not draw a clear conclusion whether intravenous anaesthesia maintenance is better that the inhalational one at preventing delirium (85). Still, taking into consideration the low grade evidence, they found no difference in the incidence of postoperative delirium between the different volatile anaesthetic maintenance agents. On the other hand, they found low grade evidence that maintenance with propofol based total intravenous anaesthesia may reduce postoperative delirium rates. Another recent study not included in the aforementioned review found that a higher incidence of postoperative delirium was present in the Desflurane group compared to the Isoflurane one in older surgical patients (86). A multicenter, open-label, randomised controlled trial on 1200 elderly patients undergoing cancer surgery is underway and is studying the impact of inhalational vs intravenous anesthesia on early delirium and long-term survival (87).
F)Pain management
Pain is another important factor implicated in the emergence of postoperative delirium and more so, it is possible to be influenced. A systematic review on the topic of pain management for hip fracture found only nerve blocks to be effective in reducing the incidence of postoperative delirium and qualified it as a moderate strength of evidence. There was insufficient evidence on preventing delirium through pain-management with spinal anaesthesia alone, in continuous administration through a spinal catheter, or in conjunction with other medications such as morphine (88).
G)Dexmedetomidine
This is a drug that has sedative, anxiety reducing and pain decreasing effects through its highly selective alpha2- adrenergic agonistic action. It induces sedation by lowering the activity of noradrenergic neurons in the locus ceruleus and tuberomammilary nucleus, thereby increasing the activity of inhibitory GABA neurons in the ventrolateral preoptic nucleus (89). Although the use of dexmedetomidine has had mixed evidence regarding its efficacy in preventing delirium in surgical non-ICU patients, two recent 2018 meta-analyses shed light on the matter and be in accord that its perioperative administration can reduce delirium rates in both cardiac and non-cardiac surgical patients (90) (91). More studies are needed to determine the optimal dosage, safety profile (it might increase the rate of bradycardia) and timing, as one paper stated that intraoperative infusion in major elective non-cardiac surgery did not significantly reduce the incidence of delirium compared to placebo (92).
ENDING NOTES
The topic of postoperative delirium is far from being over- researched. On the contrary, it can be stated that, in fact, too few people (medical staff, healthcare management and family members alike) know of this entity. A common, united effort of all medical and surgical specialists involved is needed to spread the knowledge on the topic and further the current research on all levels – basic sciences, translational and clinical.
REFERENCES:
1.Thorsteinsdóttir SA, Sveinsdóttir H, Snædal J. . Delirium after open cardiac surgery: systematic review of prevalence, risk factors and consequences. Laeknabladid 2015; 101(6):305-11
2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383:911–922
3.Kyziridis TC. Post-operative delirium after hip fracture treatment – a review of the current literature. GMS Psycho-Social Medicine 2006; 3:Doc01.
4.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010; 304(4):443-51
5.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168: 27–32
6.Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007; 19(2):197–214. Epub 2006 Sep 14.
7.Sockalingam S, Parekh N, Bogoch II, et al. Delirium in the postoperative cardiac patient: a review. J Card Surg 2005; 20(6):560–7
8.Milstein A, Pollack A, Kleinman G, Barak Y. Confusion/delirium following cataract surgery: an incidence study of 1-year duration. Int Psychogeriatr 2002; 14(3):301-6.
9.Xue P, Wu Z, Wang K, Tu C, Wang X. Incidence and risk factors of postoperative delirium in elderly patients undergoing transurethral resection of prostate: a prospective cohort study. Neuropsychiatr Dis Treat. 2016; 12:137-42.
10.Wass S, Webster PJ, Nair BR. Delirium in the Elderly: A Review. Oman Med J 2008; 23(3): 150–157.
11.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM .Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49(5):516-22
12.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340(9):669-76.
13.O’Neal JB, Shaw AD. Predicting, preventing, and identifying delirium after cardiac surgery. Perioper Med (Lond) 2016; 5: 7.
14.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013;
21(12):1190–222
15.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996; 275(11):852–7
16.Oldroyd C, Scholz AFM, Hinchliffe RJ, McCarthy K, Hewitt J, Quinn TJ. A systematic review and meta-analysis of factors for delirium in vascular surgical patients. J Vasc Surg 2017; 66(4):1269-1279.
17.Tanaka M, Tani N, Maruo T, et al. Risk factors for postoperative delirium after deep brain stimulation surgery for Parkinson Disease. World Neurosurg 2018; 114:518-523.
18.Zhang DF, Su X, Meng ZT, et al. Preoperative severe hypoalbuminemia is associated with an increased risk of postoperativedelirium in elderly patients: Results of a secondary analysis. J Crit Care 2018; 44:45-50.
19.Zhang H, Tang Y, Qin Y. Risk factors and outcomes of postoperative delirium in colorectal cancer patients over 60 years. Zhonghua Wei Chang Wai Ke Za Zhi 2017; 20(11):1263-1268
20.Kyeong S, Shin JE, Yang KH, Lee WS, Chung TS, Kim JJ. Neural predisposing factors of postoperative delirium in elderly patients with femoral neck fracture. Sci Rep 2018; 8(1):7602.
21.Hall RJ, Watne LO, Idland AV, et al. Cerebrospinal fluid levels of n e o p t e r i n a r e e l e v a t e d i n d e l i r i u m a f t e r h i p f r a c t u r e . J Neuroinflammation 2016; 13(1):170.
22.Osse RJ, Fekkes D, Tulen JH, et al. High preoperativeplasma neopterin predicts delirium after cardiac surgery in older adults. J Am Geriatr Soc 2012 (4):661-8.
23.Vasunilashorn SM, Dillon ST, Inouye SK, et al. High C-Reactive protein predicts delirium incidence, duration, and feature severity after major noncardiac surgery. J Am Geriatr Soc 2017; 65(8):109-116.
24.Witlox J, Kalisvaart KJ, de Jonghe JF, et al. Cerebrospinal fluid β- amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc 2011;
59(7):1260-7.
25.Cunningham EL, McGuinness B, McAuley DF, et al. CSF Beta- amyloid 1-42 concentration predicts delirium following elective arthroplasty surgery in an Observational Cohort Study. Ann Surg 2018; doi: 10.1097/SLA.0000000000002684
26.Price CC, Garvan C, Hizel LP, Lopez MG, Billings FT 4th. Delayed recall and working memory MMSE domains predict delirium following cardiac surgery. J Alzheimers Dis 2017; 59(3):1027-1035.
27. Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium? value of bedside instruments. JAMA 2010; 304(7):779- 86.
28.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry 1983; 140(6):734-9
29.Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 2005; 29(4):368-75
30.Detroyer E, Clement PM, Baeten N, et al. Detection of delirium in palliative care unit patients: a prospective descriptive study of the Delirium Observation Screening Scale administered by bedside nurses. Palliat Med 2014; 28(1):79-86.
31.Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014; 43(4):496-502.
32.O’Regan NA, Ryan DJ, Boland E, et al. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry 2014; 85(10):1122-31.
33.Ryan DJ, O’Regan NA, Caoimh RÓ, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open
2013; 3(1). pii: e001772. doi: 10.1136/bmjopen-2012-001772
34.Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin 2015; 33(3): 505–516
35.Guo Y, Sun L, Li L, et al. Impact of multicomponent, nonpharmacologic interventions on perioperative cortisol and melatonin levels and postoperative delirium in elderly oral cancer patients. Arch Gerontol Geriatr 2016; 62:112-7.
36.Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017; 96(26):e7361.
37.Chen CC, Li HC, Liang JT, et al. Effect of a Modified Hospital Elder Life Program on Delirium and Length of Hospital Stay in Patients JAMA Surg 2017 ;152(9):827-834
38.Siddiqi N1, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev
2016; 3:CD005563. doi: 10.1002/14651858.CD005563.pub3
39.Oberai T, Laver K, Crotty M, Killington M, Jaarsma R. Effectiveness of multicomponent interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review. Int Psychogeriatr 2018; 30(4):481-492.
40.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program : Systematic Review and Meta-analysis of Effectiveness. Am J Geriatr Psychiatry 2018; pii: S1064-7481(18)30373-7.
41.Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE study. Intern Med J 2007; 37(2):95-100
42.Martinez FT, Tobar C, Beddings CI, Vallejo G, Fuentes P. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing 2012; 41(5):629-34.
43.Mailhot T, Cossette S, Côté J, et al. A post cardiac surgery intervention to manage delirium involving families: a randomized pilot study.
Nurs Crit Care 2017; 22(4):221-228.
44.van der Zanden V, Beishuizen SJ, Swart LM, de Rooij SE, van Munster BC.
The Effect of Treatment of Anemia with Blood Transfusion on Delirium: A Systematic Review. J Am Geriatr Soc 2017; 65(4):728- 737.
45.van der Zanden V, Beishuizen SJ, Scholtens RM, de Jonghe A, de Rooij SE, van Munster BC. The Effects of Blood Transfusion on Delirium Incidence.
J Am Med Dir Assoc 2016; 17(8):748-53.
46.Motaharinia J, Etezadi F, Moghaddas A, Mojtahedzadeh M. Immunomodulatory effect of hypertonic saline in hemorrhagic shock. Daru 2015; 23:47.
47.Xin X, Xin F, Chen X, et al. Hypertonic saline for prevention of delirium in geriatric patients who underwent hip surgery.
J Neuroinflammation 2017; 14(1):221.
48.Xin X, Huo SP, Zhang Q, Li YN, Wang L, Wang QJ. Effects of preconditioning with hypertonic saline solution on postoperative delirium in the aged. Zhonghua Yi Xue Za Zhi. 2017; 97(39):3072-3078.
49.Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Preoperative exercise capacity is associated with the prevalence of postoperative delirium in elective cardiac surgery. Aging Clin Exp Res 2018; 30(1):27- 34
50.Abraha I, Rimland JM, Trotta F, et al. Non-Pharmacological Interventions to Prevent or Treat Delirium in Older Patients: Clinical Practice Recommendations The SENATOR-ONTOP Series. J Nutr Health Aging 2016;20(9):927-936
51.Abraha I, Trotta F, Rimland JM, et al. Efficacy of Non- Pharmacological Interventions to Prevent and Treat Delirium in Older Patients: A Systematic Overview. The SENATOR project ONTOP Series. PLoS One 2015; 10(6):e0123090.
52.Serafim RB, Bozza FA, Soares M, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: A systematic review. J Crit Care 2015; 30(4):799-807.
53.Santos E, Cardoso D, Neves H, Cunha M, Rodrigues M, Apóstolo J. Effectiveness of haloperidol prophylaxis in critically ill patients with a high risk of delirium: a systematic review. JBI Database System Rev Implement Rep 2017; 15(5):1440-1472.
54.Teslyar P, Stock VM, Wilk CM, Camsari U, Ehrenreich MJ, Himelhoch S. Prophylaxis with Antipsychotic Medication Reduces the Risk of Post-Operative Delirium in Elderly Patients: A Meta-Analysis. Psychosomatics 2013:54:124–131
55.Fukata S, Kawabata Y, Fujisiro K, et al. Haloperidol prophylaxis does not prevent postoperative delirium in elderly patients: a randomized, open-label prospective trial. Surg Today 2014; 44(12):2305-13.
56.Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2018; 6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
57.Schrijver EJ, de Graaf K, de Vries OJ, Maier AB, Nanayakkara PW.
Efficacy and safety of haloperidol for in-hospital delirium prevention and treatment: A systematic review of current evidence. Eur J Intern Med
2016; 27:14-23.
58.Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87(7):767-74
59.Schrijver EJ, Verstraaten M, van de Ven PM, et al. Low dose oral haloperidol does not prolong QTc interval in older acutely hospitalised adults: a subanalysis of a randomised double-blind placebo-controlled study. J Geriatr Cardiol 2018; 15(6):401-407
60.Kluger C, Shah P, Maiti S, Babalola O, Mulvany C, Sinvani L. Therapeutic advances in the prevention and treatment of delirium in the hospital setting. American Journal of Therapeutics 2018; 25:3–14.
61.Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics 2010; 51(5):409-18.
62.Jain FA, Brooks JO 3rd, Larsen KA, et al. Individual risk profiles for postoperative delirium after joint replacement surgery. Psychosomatics
2011; 52(5):410-6.
63.Nazemi AK, Gowd AK, Carmouche JJ, Kates SL, Albert TJ, Behrend CJ. Prevention and Management of Postoperative Delirium in Elderly Patients Following Elective Spinal Surgery. Clin Spine Surg 2017;
30(3):112-119.
64.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care 2007; 35(5):714-9.
65.Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res
2011; 71(4):277-81.
66.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MINDrandomized, placebo-controlled trial. Crit Care Med 2010;
38(2):428-37.
67.Rivière J, van der Mast RC, Vandenberghe J, Van Den Eede F. Efficacy and Tolerability of Atypical Antipsychotics in the Treatment of Delirium: A Systematic Review of the Literature. Psychosomatics 2018; pii: S0033-3182(18)30258-5.
68.Pintor L, Fuente E, Bailles E, Matrai S. Study on the efficacy and tolerability of amisulpride in medical/surgical inpatients with delirium admitted to a general hospital. Eur Psychiatry 2009;24(7):450-5.
69.Kirino E. Use of aripiprazole for delirium in the elderly: a short review. Psychogeriatrics 2015;15(1):75-84.
70.Chen S, Shi L, Liang F, et al. Exogenous Melatonin for Delirium Prevention: a Meta-analysis of Randomized Controlled Trials. Mol Neurobiol 2016; 53(6):4046-4053
71.Artemiou P, Bily B, Bilecova-Rabajdova M, et al. Melatonin treatment in the prevention of postoperative delirium in cardiac surgery patients. Kardiochir Torakochirurgia Pol 2015; 12(2):126-33.
72.Miyata R, Omasa M, Fujimoto R, Ishikawa H, Aoki M. Efficacy of Ramelteon for delirium after lung cancer surgery. Interact Cardiovasc Thorac Surg 2017; 24(1):8-12.
73.Booka E, Tsubosa Y, Matsumoto T, et al. Postoperative delirium after pharyngolaryngectomy with esophagectomy: a role for ramelteon and suvorexant. Esophagus 2017; 14(3):229-234.
74.Tamura K, Maruyama T, Sakurai S. Preventive effect of suvorexant for postoperative delirium after coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 2018. doi: 10.5761/atcs.oa.18-00038
75.Choy SW, Yeoh AC, Lee ZZ, Srikanth V, Moran C. Melatonin and the Prevention and Management of Delirium: A Scoping Study. Front Med (Lausanne) 2017; 4: 242. Published online 2018
76.Balan S, Leibovitz A, Zila SO, et al. The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci 2003; 15(3):363-6
77.Vural EM, van Munster BC, de Rooij SE. Optimal dosages for melatonin supplementation therapy in older adults: a systematic review of current literature. Drugs Aging 2014; 31(6):441-51.
78.Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery–a randomized controlled trial. Crit Care Med 2009; 37(5):1762-8.
79.Youn YC, Shin HW, Choi BS, Kim S, Lee JY, Ha YC. Rivastigmine patch reduces the incidence of postoperative delirium in older patients with cognitive impairment. Int J Geriatr Psychiatry 2017; 32(10):1079- 1084.
80.Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc 2011; 59 Suppl 2:S282-8.
81.Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double- blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry 2007; 22(4):343-9.
82.Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev 2018;
6:CD012494. doi: 10.1002/14651858.CD012494.pub2.
83.Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev 2008; (1):CD005317. doi:
10.1002/14651858.CD005317.pub2.
84.Yokota H, Ogawa S, Kurokawa A, Yamamoto Y. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci 2003; 57(3):337-9.
85.Miller D1, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane D a t a b a s e S y s t R e v 2 0 1 8 ; 8 : C D 0 1 2 3 1 7 . d o i : 10.1002/14651858.CD012317.pub2.
86.Kinjo S, Lim E, Magsaysay MV, Sands LP, Leung JM; Perioperative Medicine Research Group. Volatile anaesthetics and postoperative delirium in older surgical patients-A secondary analysis of prospective cohort studies. Acta Anaesthesiol Scand 2018. doi: 10.1111/aas.13227
87.Zhang Y, Li HJ, Wang DX, et al. Impact of inhalational versus intravenous anaesthesia on early delirium and long-term survival in elderly patients after cancer surgery: study protocol of a multicentre, open-label, and randomised controlled trial. BMJ Open 2017; 7(11):e018607.
88.Abou-Setta AM, Beaupre LA, Rashiq S, et al. Comparative effectiveness of pain management interventions for hip fracture: a systematic review. Ann Intern Med 2011; 155(4):234-45.
89.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2- adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003; 98(2):428-36.
90.Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV,Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth 2018; 121(2):384-397.
91.Wu M, Liang Y, Dai Z, Wang S.Perioperative dexmedetomidine reduces delirium after cardiac surgery: A meta-analysis of randomized controlled trials. J Clin Anesth 2018; 50:33-42.
92.Deiner S, Luo X, Lin HM, et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg 2017; 152(8):e171505.
***




