A non-interventional study to observe real-life usage of atypical antipsychotics in the acute inpatient management of schizophrenia in Central and Eastern Europe
Abstract
Background:Desi majoritatea ghidurilor terapeutice recomandă utilizarea antipsihoticelor atipice (AA) ca monoterapie în managementul episodului psihotic acut al pacienților cu schizofrenie, atât în SUA precum si în Europa Occidentală, deseori acesti pacienți sunt tratați cu antipsihotice de primă generație, combinație de antipsihotice (polifarmacie) ori doze subterapeutice de AA. Comparativ, există mai puține date referitoare la managementul acestor pacienți în Europa Centrală si de Est. Obiective: Evaluarea discrepanțelor între practica clinică si ghidurile terapeutice în managementul episodului psihotic acut la pacienții cu schizofrenie. Metodă: Studiu non-intervențional, multinațional, multicentric (RECONNECT-S GAMMA) în scopul descrierii managementului episodului psihotic acut la pacienții cu schizofrenie spitalizați pentru un astfel de episod. Pacienții studiați (vârsta ≥ 18 ani) au avut o vizită de studiu în ziua externării. Au fost colectate date demografice si date referitoare la tratamentul antipsihotic si medicația concomitentă administrate în perioada spitalizării precum si recomandările la externare. Rezultate: Din 496 pacienți, 49 (9,9%) au fost tratați cu AA în monoterapie si 418 (84,3%) au fost tratați cu terapie de combinație. Pe ansamblu cele mai folosite medicamente au fost quetiapina orală (n=129; 27,4%), risperidona orală (n=95; 20,2%), olanzapina orală (n=104; 22,1%) si clozapina orală (n=73; 15.5%), cu variații de administrare între țări. La externare unui număr semnificativ (n=373; 75.2%) de pacienți (n=373; 75,2%) i-a fost prescrise AA ca medicație de întreținere, un număr de 335 (69.9%) primind aceeasi medicație cu care au fost tratați pe perioada spitalizării. Concluzii: Practica clinică obisnuită în Eudopa Centrală si de Est diferă față de ghidurile terapeutice, un număr mai mic de pacienți cu schizofrenie primind AA ca monoterapie pentru episodul psihotic acut.
Introduction
Schizophrenia is a severe form of mental illness, which has been estimated by the World Health Organization to affect approximately 24 million people worldwide (1). Although there are relatively few new cases of schizophrenia diagnosed each year, the prevalence is high. A review of studies reporting prevalence of schizophrenia, that included data from 46 countries, revealed median values, per 1,000 persons for the distribution of prevalence estimates, of 4.6, 3.3 and 4.0 for point (21 studies), period (34 studies) and lifetime (24 studies) prevalence of schizophrenia (2). Prevalence data in Romania has not been recorded since 1987, when the lifetime prevalence of schizophrenia in an urban environment was reported as 10.1 per 1,000 (3). Furthermore, the schizophrenia prevalence rate in 2011 was documented as 9.4 per 1,000 in Latvia (4).The disease burden tends to be much lower in most of Europe, the USA, Australia and Japan compared to countries in Eastern Asia, Oceania and the Middle East (5). In 2004, the age-standardised disability-adjusted life years (DALY), per 100,000 population, attributable to schizophrenia range from 322 in Indonesia to 164 in Australia, whilst Hungary, Latvia and Romania have estimates of 207, 204 and 237, respectively (6).
Patients diagnosed with schizophrenia present with a range of symptoms including long duration, bizarre delusions, negative symptoms, and non-affective psychosis (7). Over 50% of patients fail to receive appropriate care, and therefore often have symptoms which persist for long periods of time (1). Long-term administration of antipsychotic medication has been shown to be the most effective strategy to improve the prognosis of schizophrenia (8). However, a systematic review of 39 studies reported a 41% mean rate of antipsychotic non-adherence in schizophrenia (9); this leads to exacerbations and relapses in patients that often require hospitalisation. A significant challenge for clinicians in the management of patients with schizophrenia is the occurrence of acute psychotic episodes (10); however, there is limited information relating to the use of antipsychotics on the agitation component during these episodes in Central and Eastern Europe.
In Hungary, physicians follow the Ministry of Health’s guidelines (edited and compiled by the Hungarian Psychiatric Professional College) for the treatment of schizophrenia. Although treatment recommendations have been published by the Latvian Psychiatric Association (11), they are solely recommendations and therefore not imposed on psychiatrists. In both Latvia and Romania, the treatment approach to schizophrenia is very much in line with internationally recognised guidelines, including those of the American Psychiatric Association (APA) and National Institute for Health and Care Excellence (NICE). Guidelines published by the APA recommend that selection of an antipsychotic agent be guided by the patient’s past medication history, current symptoms, and comorbidities, other concurrent treatments, and preferences –(1213). The guidelines state that, for schizophrenic patients in the acute phase, second- generation antipsychotic monotherapy should be considered as the first-line option; this recommendation is based on the decreased risk of extrapyramidal side effects and tardive dyskinesia associated with second-generation compared with first-generation agents –(1213).
Despite the guidelines usually recommending a preference for atypical antipsychotic (AAP) monotherapy during acute psychotic episodes in schizophrenic patients, evidence shows that first-generation antipsychotics, polypharmacy, intramuscular route of administration, and AAP at lower than recommended doses are being frequently used to treat acute episodes (8). For example, in Hungary a study conducted at the Semmelweis University in Budapest and the Galfi Bela HC Institution in Pomaz, revealed that although AAP use increased from 1998 to
2002, typical antipsychotics were still used in clinical practice (14). A more recent study investigating the prescribing pattern of psychotropic drugs to 1,304 patients in eight psychiatric hospitals in Albania, Croatia, Macedonia, Serbia and Romania reported that only 6.8% of patients received AAP as monotherapy, whilst 26.5%,
42.1% and 22.1% received them in combination with two, three or four other antipsychotic medications, respectively (15). Overall, only 40% of patients were prescribed AAP, while 63% received typical antipsychotics (15). This suggests that in addition to guidelines, physicians use their discretion and experience when determining the most appropriate treatments for their patients. Also since typical antipsychotics are cheaper than AAP (16), cost and availability could be additional factors they consider. There is a need, therefore, for further research to examine the discrepancies between current clinical practice and g u i d e l i n e r e c o m m e n d a t i o n s . T h e a i m o f t h e RECONNECT-S GAMMA study was to describe the real- life usage of AAP in the acute inpatient management of schizophrenia, in order to gain an understanding of current treatment practices and identify any disparities that may exist between these and guideline recommendations in Central and Eastern Europe.
Methods
Study Design
This was a non-interventional, multicentre study, registered as NCT01491412, to describe the management of subjects with schizophrenia who were hospitalised due to an acute psychotic episode. Thirty-three investigators participated in the study: 15 in Romania, 10 in Latvia and eight in Hungary. For the purpose of serving the study objectives, selection of participating sites aimed to ensure that the schizophrenic patient population of the three countries was accurately represented. Sites throughout the three countries were included that provide specialised care to a wide range of schizophrenic patients, including patients who have recently been diagnosed, patients with chronic disease history, patients with difficult-to-treat schizophrenia, and patients who require tranquillisation. A total of 496 study subjects were recruited; 211 from Romania, 183 from Hungary and 102 from Latvia.
Subjects attended one visit on the day of their discharge after hospitalisation due to an acute psychotic episode; during this visit data on demographics, diagnosis, and medical history were recorded,as shown in fig. 1. In addition, data on antipsychotic treatment and concomitant medication were collected for the hospitalisation period, together with the recommendation at discharge.
Study Subjects
Subjects were eligible for enrolment in the study if they were 18 years or older, met the diagnostic criteria for schizophrenia stated in The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), and were hospitalised due to an acute psychotic episode. The study required that the subject had the ability to understand and comply with the requirements of the study, as judged by the investigator. Written informed consent was obtained from the subject and/or his/her legal representative (as per local regulatory requirements). Ethical approval was obtained for each study site in Hungary and Latvia. Only health authority approval was required in Romania; this was obtained from the National Medicine and Medical Devices Agency.
Exclusion criteria consisted of current participation in a clinical trial or previous enrolment in the current study (in the case of re-hospitalisation).
Study Objectives
The primary objective of the study was to describe the use of AAP in subjects with schizophrenia during hospitalisation due to an acute psychotic episode, by evaluation of drug, dose, mode and length of administration of the medication.
Secondary objectives included: evaluation of the use of AAP as monotherapy and the use of combinations of antipsychotics during the hospitalisation period; investigation of the main criteria used for selection of an antipsychotic to treat acute episodes of schizophrenia; description of the use of psychometric scales to evaluate the disease symptoms during the hospitalisation period; investigation of the use of concomitant psychiatric medications (other than atypical antipsychotics) during hospitalisation; the correlation between antipsychotic medication used during hospitalisation and maintenance therapy recommended upon discharge.
Safety
This non-interventional study was designed without a specific safety objective, therefore no safety data were pro-actively collected. Spontaneously-mentioned safety events were reported as required by post-marketing pharmacovigilance regulations.
Statistical Analyses
A descriptive analysis approach (including frequency tables) was used owing to the non-interventional design of the study. As appropriate, a two-sided 95% confidence interval was obtained for the population estimation of the variables. All calculations and summaries were produced using SAS Version 9.2 (SAS 2009), with medications coded using the WHO drug dictionary, version 12.1.
Results
Patient demographics and baseline characteristics
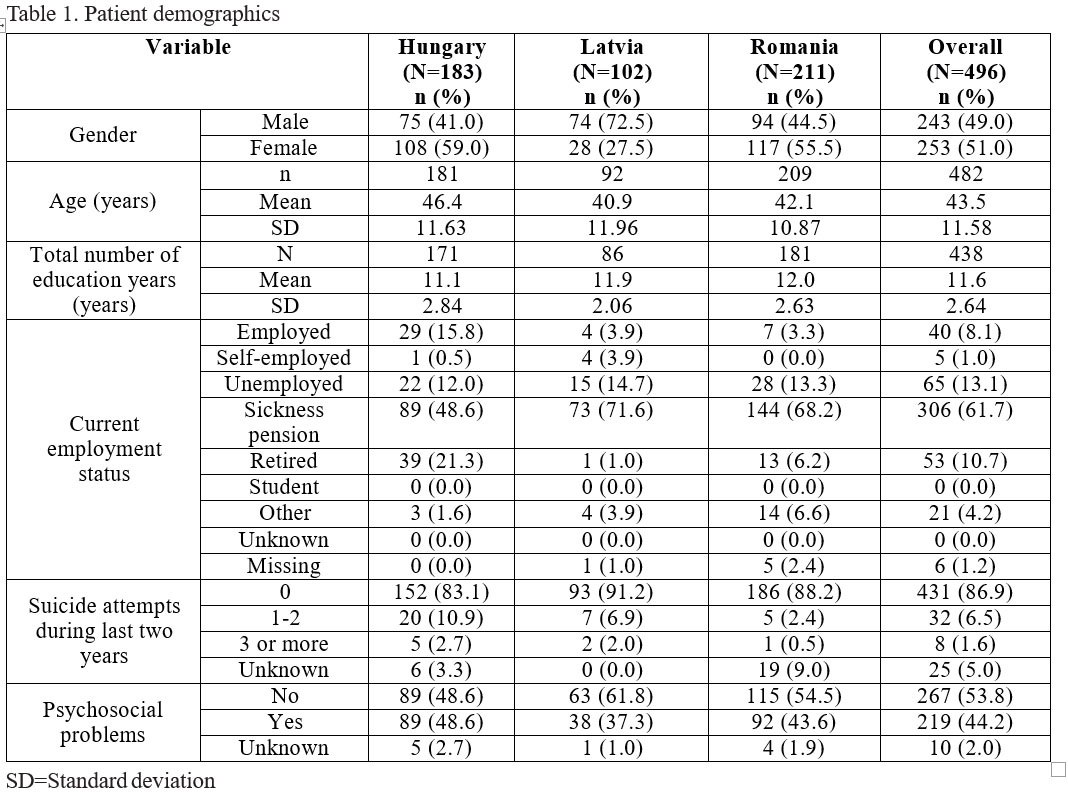
The study was conducted between December 2011 and May 2012. Patient demographics are shown in Table 1. Of the 496 subjects enrolled in the study, all met the inclusion criteria and none of the exclusion criteria applied to these subjects; 243 participants (49.0%) were male. Participants had a mean age ± standard deviation (SD) of 43.5 ± 11.58 years, and a mean number of years of education ± SD of
11.6 ± 2.64. All subjects were Caucasian (race data missing for one subject) and the majority were in receipt of sickness pension (61.7%), unemployed (13.1%), or retired (10.7%). Over 40% of study subjects reported psychosocial problems and approximately 8% had made one or more suicide attempt(s).
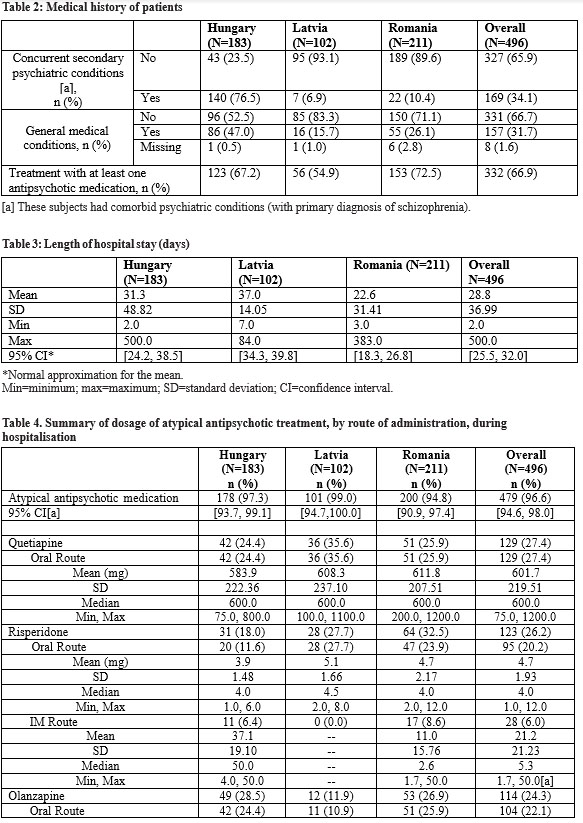
Overall, 34.1% of patients had concurrent psychiatric conditions in addition to their primary schizophrenia diagnosis (Table 2). In Hungary, 76.5% of patients were reported to have a concomitant psychiatric condition, compared with just 6.9% and 10.4% of patients from Latvia and Romania, respectively. One hundred and fifty seven subjects (31.7%) had other general medical conditions: 46 (9.3%) had hypertensive diseases, 34 (6.9%) had diseases of the digestive system, 28 (5.6%) had obesity and other hyperalimentation comorbidities, while 23 (4.6%) had diabetes mellitus. Prior to enrolment, 332/496 subjects (66.9%) were being treated with at least one antipsychotic medication. The most frequently reported antipsychotic agents were: risperidone (16.5%); quetiapine (15.5%); and olanzapine (12.5%).
Primary objectives
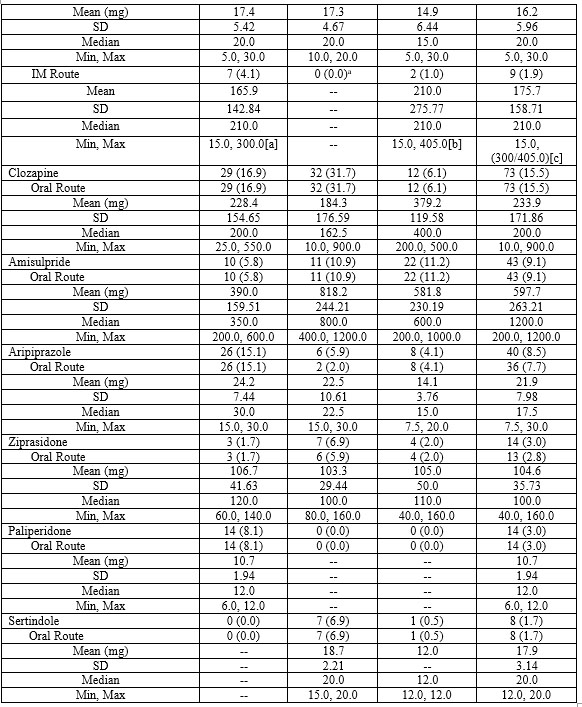
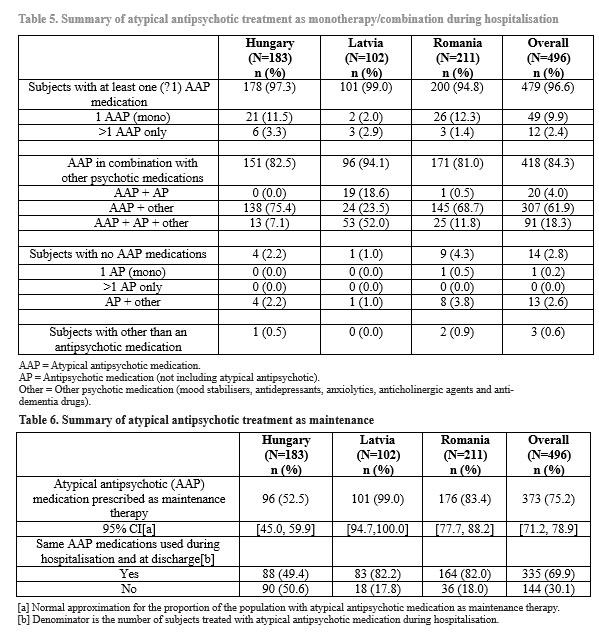
Length of hospital stay varied between countries with the shortest mean hospital stays being observed in Romania followed by Hungary and Latvia (22.6, 31.3 and 37.0 days, respectively) (Table 3). The majority of subjects (479/496, 96.6%) were treated with at least one AAP (Table 4), the most frequently prescribed were quetiapine (27.4%), oral risperidone (20.2%) and oral olanzapine (22.1%). A smaller percentage of subjects received oral aripiprazole (7.7%), intramuscular risperidone (6.0%) or intramuscular olanzapine (1.9%). In addition, 14/496 study subjects (2.8%) did not receive treatment with an AAP, and 3/496 study subjects (0.6%) received something other than an antipsychotic medication. Variation was observed between daily doses of AAP most commonly administered in different countries. The highest average daily dose for oral risperidone was observed in Latvia and the lowest in Hungary. While the highest and lowest observed average daily doses of oral olanzapine were in Hungary and Romania, respectively, the reverse was observed for oral quetiapine, with daily doses being lowest in Hungary and highest in Romania.
Secondary objectives
Use of monotherapy or combination therapy and concomitant psychiatric medication
Although the majority of subjects (479, 96.6%) received at least one AAP during hospitalisation, only 49/479 subjects (9.9%) received AAP as monotherapy, while 12/479 subjects received more than one AAP (Table 5). Overall,
418 subjects (84.3%) were treated with AAP in combination with other psychiatric medications: 20 (4.0%) with a combination of atypical and typical antipsychotics, 307 (61.9%) with an AAP and another psychiatric medication, and 91 (18.3%) with an AAP, a typical antipsychotic and another psychiatric medication (Table 6). Of the 418 (84.3%) subjects who received at least one concomitant psychiatric medication: 267 (53.8%) were treated with antiepileptics; 180 (36.3%) with anxiolytics; 125 (25.2%) with antipsychotics; and
101 (20.4%) with antidepressants.
Criteria used for selection of an antipsychotic
The most frequently stated rationales for the selection of the AAP medication chosen were medication history (41.9%) and current symptoms (43.8%).
Use of psychometric scales to evaluate patients’ symptoms
The majority of study subjects (93.3%) were evaluated in accordance with the clinical experience of the consulting physician; psychometric scales were not used to evaluate disease symptoms during hospitalisation for any of the subjects enrolled in this study.
Maintenance therapy upon discharge
Upon discharge, most study subjects (n=373; 75.2%) were prescribed AAP as maintenance therapy (Table 6). However, the prescription pattern was different for Hungary, with only 52.5% prescribed AAP, compared with 99.0% and 83.4% in Latvia and Romania, respectively. Overall, of the 479 subjects treated with an AAP during hospitalisation, 335 (69.9%) were prescribed the same medication(s) at discharge that they had received during hospitalisation.
Discussion
This study provides evidence for the type, dose, mode and length of administration of AAP used in the management of acute psychotic episodes in subjects with schizophrenia in Central and Eastern Europe. There was a significant d i f f e r e n c e o b s e r v e d b e t w e e n t h e t r e a t m e n t recommendations for AAP monotherapy contained within the guidelines – (1213) and current real-life clinical practice, where the use of polypharmacy is common. A large observational study of the outcomes of antipsychotic treatment for schizophrenia in Europe reported by Haro and colleagues demonstrated that combination therapy is commonly used in schizophrenia (8). The findings of RECONNECT-S GAMMA support this observation, with just 49/496 individuals receiving AAP as monotherapy for an acute episode of schizophrenia, while the vast majority (418/496, 84.3%) of study subjects received combination therapy with AAP and one or more additional psychotic medication(s). Various factors exist which may affect the choice of medication prescribed to schizophrenic patients. For example, in Romania, since the patient is usually treated in hospital, the medication administered by the physician is greatly influenced by the availability of drugs in the hospital pharmacy. Lack of availability could prevent psychiatrists from being able to select a medication which they feel is most appropriate for their patient based on their clinical knowledge.
Although the majority of subjects received AAP as combination therapy in Hungary and Romania the majority of additional medications administered were other psychiatric medications and included antiepileptics, anxiolytics and antidepressants, not only typical antipsychotics. We suggest that according to historic tradition, mood stabilisers and anxiolytics were used to manage patients’ symptoms in order to improve their clinical outcomes and social functioning (17). The reduced use of typical antipsychotics, however, is promising since they are associated with an increased risk of extrapyramidal side-effects and tardive dyskinesia (13). In Latvia, the use of typical antipsychotics was far higher, with 18.6% receiving atypical antipsychotics in combination with typical antipsychotics, and 52.0% receiving both atypical and typical antipsychotics in combination with other additional psychiatric medications. This could potentially be because the existing reimbursement system and allocated finances only permit 60% of schizophrenic outpatients to receive AAP. This is taken into consideration when patients are treated during their stay in hospital, and rather than switching from an atypical to a typical antipsychotic at discharge, physicians decide at treatment initiation which patients should be treated with a typical or atypical antipsychotic.
T h e o u t p a t i e n t t r e a t m e n t o f i n d i v i d u a l s w i t h schizophrenia is challenging owing to the high frequency of missed appointments, resistance to treatment and low adherence to medication, that are frequently observed in this patient population –(1819). Monotherapy with AAP is the recommended first-line strategy (12,13,20); however, no single agent is suitable for the universal treatment of all patients. Therefore, an individualised treatment strategy is frequently applied, whereby the agent prescribed is selected according to factors such as physicians’ previous experience and patient response to medication (8). This approach may ultimately result in the application of polypharmacy as a treatment approach which can be difficult to study in randomised controlled clinical trials (21).
There were some differences in the study populations between the three countries included within the study. While a similar percentage of the study population was male in Hungary and Romania (41.0% and 44.5%, respectively), the percentage of male participants was much greater in Latvia (72.5%). Although there are no major gender differences in morbidity with schizophrenia in Latvia, psychiatric departments are segregated between male and female patients, and the imbalance is attributed to a greater proportion of physicians in the male departments taking part in the study. The mean age of participants in Hungary (46.4 years) was greater than the mean ages of participants in Romania and Latvia (42.1 and
40.9 years, respectively). Similarly, the percentage of study participants who were retired was greater in Hungary (39%, presumed due to early retirement perhaps due to disability) compared to Latvia (1%) and Romania (13%). In Hungary the percentage of people in receipt of sickness benefit (48.6%) was lower than in Latvia and Romania (71.6% and 68.2%, respectively), and potentially occurred because there was a greater number of people in employment (29% of participants in Hungary were employed compared with 4% in Latvia and 7% in Romania).
A number of inter-country differences in comorbidities were also observed between the three countries. For example, 76.5% of study subjects from Hungary reported having a concurrent psychiatric condition (other than schizophrenia) compared with 6.9% from Latvia and
10.4% from Romania. These differences may be accounted for by systematic screening for these comorbidities in Hungary compared with Latvia or Romania. The average daily dose for oral risperidone was highest for Latvia and lowest for Hungary, with the highest average daily dose of oral olanzapine administered in Hungary and lowest in Romania. We suggest that the average dose variation reflected the severity of individual patients’ cases of schizophrenia, psychiatrists’ personal experience with administration of the medication, and evaluation of the benefit-risk ratio to their patient. The average length of hospital stay also varied between countries; it was shortest in Romania and longest in Latvia. In Romania hospitals are under a great deal of administrative pressure to shorten the length of stay of patients in psychiatric hospitals; arguments for this are derived from US literature which describes movement towards hospitalisation durations of 9–10 days for acute psychotic episodes (22). This could therefore in part account for mean hospitalisation stays being considerably shorter in Romania than the other two countries.
Mean AAP dose recommendations were followed by physicians in all three countries in this study. A similar proportion of patients from each country received at least one AAP prior to hospitalisation: 123 (67.2%) subjects in Hungary; 56 (54.9%) in Latvia; and 153 (72.5%) in Romania. Additionally, 53.8% of patients received antiepileptic therapy and 20.4% were treated with antidepressants. The relatively high rate of use of these agents may be because of symptomatic motives such as mood, aggressive behaviour or impulsivity in patients.
Post-discharge prescription patterns in Romania and Latvia were highly similar to the hospitalisation prescription pattern but were markedly different in Hungary; in Latvia and Romania 99.0% and 83.4% of patients respectively, were prescribed the same medication at discharge as during hospitalisation, while in Hungary only 52.5% were. In Romania it is customary to continue in-hospital medication patterns upon discharge unless there is good reason to add or remove a medication. As a result, prescription patterns would generally not be expected to differ. However in Hungary, on discharge patients commonly return to their own doctor, who may then use his or her own personal experience to determine the appropriate treatment once the acute episode has been controlled.. This can lead to a change in patients’ medication in terms of antipsychotic and/or dose, or switching to monotherapy. These findings illustrate further how individualised treatment strategies and common clinical practice can influence the treatment of acute episodes of schizophrenia, leading to the observed differences between different countries. Given the non- randomised nature of the current study, the above findings should be interpreted conservatively, and additional studies may be required to further elucidate the prescribing practices within these countries.
In conclusion, this study indicated that although the majority of subjects with schizophrenia were treated with AAP for acute psychotic episodes during hospitalisation, they were commonly used in combination with other psychiatric medications, rather than as monotherapy. These treatment practices are contrary to current guideline recommendations and further studies to investigate the resulting outcomes for patients, and whether such disparities exist in other countries, are warranted.
Role of funding source
This study was sponsored by AstraZeneca.
Contributors
Radu Teodorescu served as International Coordinating Investigator in Romania; Gabor Feller served as National Coordinating Investigator, Hungary; Elmars Rancans served as National Coordinating Investigator, Latvia; Dan Prelipceanu served as Principal Investigator in Romania.
Author disclosures
Gábor Feller has served as adviser or speaker for Eli Lilly, Janssen-Cilag, Egis, and AstraZeneca.
Dan Prelipceanu has no conflict of interests to declare. Elmar Rancans has served as advisor or speaker for AstraZeneca, Gedeon Richter, Janssen Pharmaceuticals, Lundbeck, Sanofi-Aventis and Servier.
Radu Teodorescu has served as advisor or speaker for AstraZeneca, Bristol-Myers Squibb and Eli Lilly.
Acknowledgements
This manuscript was prepared in line with guidelines established by the International Committee of Medical Journal Editors (ICMJE) and published in its Uniform Requirements of Manuscripts Submitted to Biomedical Journals. The authors would like to thank Toby Galbraith, PhD, and Charlotte Simpson, PhD, of IMC Healthcare Communication who provided medical writing support, supported by AstraZeneca.
Abbreviations
APA=American Psychiatric Association AAP=acute atypical antipsychotic DALY=disability-adjusted life year
NICE=National Institute for Health and Care Excellence
Note: Average daily dose for subjects with dose frequency recorded as PRN (as needed) were calculated using once per day. If dose frequency was missing, once daily was assumed unless otherwise stated. If route of administration was missing, oral route was assumed.
Min=minimum; max=maximum; SD=standard deviation.
[a] Recommended maximum dose administered every 2 weeks
[b] Recommended maximum dose administered every 4 weeks
[c] Administration of 300 mg and 405 mg of olanzapine represents two different dosing schedules. Administration of
300 mg depot injection every 2 weeks (in Hungary) is equivalent to a 20 mg daily dose, whereas administration of 405 mg depot injection every 4 weeks (in Romania) is equivalent to a 10 mg daily dose.
Any patients who met the enrolment criteria (≥ 18 years and met
the diagnostic criteria for schizophrenia stated in The Diagnostic
and Statistical Manual of Mental Disorders, 4th edition (DSM- IV-TR) and had signed a written informed consent form were included in the study.
References
(1) World Health Organization. Mental Health: Schizophrenia. http://www who int/mental_health/management/
s c h i z o p h r e n i a / e n / 2 0 1 3 . A v a i l a b l e f r o m http://www.who.int/mental_health/management/schizophrenia/ en/ , accessed February 2014.
(2) McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence and mortality. Epidemiol Rev 2008; 30:67–76.
(3) Predescu V et al. Evolutia morbiditatii intr-un grup de populatie urbana. Neurologie, psihiatrie, neurochirurgie 1987; 32(1):43-59.
(4) Pulmanis T, Pelne A, Taube M. Psihiskā veselība Latvijā 2 0 11 . g a d ā Te m a t i s k a i s z i ņ o j u m s . Av a i l a b l e f r o m http://www.spkc.gov.lv/file_download/1116/Psihiska_veseliba _Latvija_2011_gada.pdf, accessed May 2014.
(5) Ayuso-Mateos JL. Global burden of schizophrenia in the year 2 0 0 0 : Ve r s i o n 1 e s t i m a t e s . A v a i l a b l e f r o m http://www.who.int/healthinfo/ statistics/bod_schizophrenia.pdf, accessed February 2014.
(6) World Health Organization (WHO). Age-standardized DALYs per 100,000 by cause, and Member State, 2004. Available from http://www.who.int/entity/healthinfo
/global_burden_disease/gbddeathdalycountryestimates2004.xl s, accessed February 2014. (7) van Os J, Kapur S. Schizophrenia. Lancet 2009; 374(9690):635-45.
(8) Haro JM, Novick D, Belger M, Jones PB. Antipsychotic type and correlates of antipsychotic treatment discontinuation in the outpatient treatment of schizophrenia. Eur Psychiatry 2006; 21(1):41-7.
(9) Lacro JP, Dunn LB, Dolder CR, Leckbend SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002; 63:892-909.
(10) Gorwood P. Meeting everyday challenges: antipsychotic therapy in the real world. Eur Neuropsychopharmacol 2006; Suppl 3:S156-62.
(11) Latvijas Psihiatru Asiciacijas. Depresijas norise un ārstēšanas iespējas Vadlīnijas (2008). Available from http://psihosomatika.lv/public/files/depresijas_vadl_2009.pdf, accessed May 2014.
(12) American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Second Edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid =1665359
(13) Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011; 25(5):567–620.
(14) Klebovich A, Bujdosó Z, Zelkó R. Utilization and comparison study of antipsychotics at the Department of Psychiatry and Psychotherapy of the Semmelweis University and the Galfi Bela Hospital. Acta Pharm Hung 2003; 73(2):131- 5.
(15) Jordanova V, Maric NP, Alikaj V, et al. Prescribing practices in psychiatric hospitals in Eastern Europe. EurPsychiatry 2011; 26(7):414-8.
(16) Luchins D. Typical Versus Atypical Antipsychotics. Am J Psychiatry 2004; 161(10):1927–1927.
(17) Predescu, V. Psihiatria. Vol I. Ed Medicala, Bucuresti, 1989. (18) Hellewell JS. Treatment-resistant schizophrenia: reviewing the options and identifying the way forward. J Clin Psychiatry 1999; 60 Suppl 23:14-9.
(19) Mitchell AJ, Selmes T. A comparative survey of missed initial and follow-up appointments to psychiatric specialties in the United Kingdom. Psychiatr Serv 2007; 58(6):868-71.
(20) Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia 7PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010; 36(1):71-93.
(21) Emborg C, Hallerback T, Jorgensen L, Carlborg A. A retrospective study of clinical usage of quetiapine XR and quetiapine IR in outpatients with schizophrenia in Denmark. Hum Psychopharmacol 2012; 27(5):492-8.
(22) Watanabe-Galloway S, Zhang W. Analysis of US trends in discharge from general hospital for episodes of severe mental illness 1995-2002. Psychiatr Serv 2007;58(4): 496-502.
***




