A REVIEW OF PERFORMANCE-BASED EXECUTIVE FUNCTION TASKS IN ADULTS WITH AUTISM AND NORMAL INTELLIGENCE
Abstract
Executive functions (EF) are a family of complex cognitive processes which are necessary to guide our thinking and behaviors towards certain goals. Research into executive functions in Autism spectrum disorder (ASD) has mainly been focusing on children and adolescents, and less on adult populations. The purpose of this article is to review the body of research that has investigated the results obtained by adults with ASD and normal levels of cognitive ability on performance-based tasks of executive functions. To this aim, we synthesized five executive functions: inhibition, cognitive flexibility, planning, working memory and generativity and the results of the studies included are presented in terms of the specific tasks employed for each. The results of the 48 studies reviewed in this article are highly variable, possibly due to the considerable differences in the methods employed, but also perhaps through a phenotypical heterogeneity of the patterns of executive functions in ASD.
Executive functions (EF) are a family of complex cognitive processes, coordinated by the frontal lobes, which are necessary to guide our thinking and behaviors towards certain goals. Executive functions help the human mind in making decisions, setting goals, organizing, planning, focusing, learning, etc. Thus, executive control is part of the adaptive mechanisms of the human mind, which we need in all the activities of everyday life.
The history of the executive function concept is closely related to the researchers’ attempts to define the functions of the frontal cortex, especially of the prefrontal cortex, as decades ago, it was considered that the executive functions are those functions that define the prefrontal cortex activity. With the progress of neuroanatomy research, the perfect equivalence between EF and PFC functions has been left aside, as it has been shown that through the many connections that the PFC establishes with other cortical and subcortical regions, it is also involved in other processes that go beyond the limits of executive control. Moreover, the numerous structural and functional neuroimaging studies conducted over the last decades have shown that executive control involves a number of other regions besides the PFC, which vary depending on the executive function in question(1).
From the first mention of a possible deficit in executive functions in autistic individuals, stated by Damsio and Maurer in 1978, to date, countless studies on populations of subjects with autism included various types of assessments of executive functions. Most of these studies focused on child and adolescent populations and only in the last decade and a half have a significant number of researches on adult populations emerged. These studies are dominated by a very high variability of the obtained results, with at least two contributing factors – the significantly different methods used (participants, executive functions analyzed and their tasks, objectives) but also a potential phenotypic heterogeneity of the executive function pattern among people with autistic spectrum disorders.
This article is intended to be a review of the research that studied the issue of executive functions (EF) in adults with autistic spectrum disorders (ASD). Thus, a search was conducted in a scientific database (Pubmed) using the following terms or their variations: “autism” or “Asperger” and “executive” and “adult”. Out of the articles that resulted from the search we excluded those in which: a) the minimum age of the subjects was less than 14 years and the mean age was less than 18 years; b) the subjects included had the Full Scale IQ <70; c) the participants had significant comorbidities such as genetic syndromes, neurological disorders, significant somatic diseases (psychiatric comorbidities were not excluded); d) the language of publication was one other than English; e) the articles were single case reports; f) the participants included had subclinical autistic features (“Broad Autism Phenotype”) or were represented by the parents or siblings of ASD patients; g) the theme presented was not relevant to this article. Thus, 71 original articles were included, out of which 51 resulted from the actual search and 20 were identified in 6 review articles on this topic (2-7).The present article is part of a more extensive paper, structured into five areas of interest which also constitute the inclusion criteria for the studies in the main paper and each of these research papers addresses at least one of the following topics: 1) the performance in executive function tasks (or the subjective evaluation) of people with ASD compared to healthy subjects or other clinical populations (i.e. schizophrenia, ADHD); 2) the relationship between EF and adult ASD symptoms or other clinical variables of interest (i.e. emotional processing or recognition, mentalization, social cognition, etc.); 3) functional neuroimaging studies with EF tasks among adult ASD patients; 4) the relationship between EF in adult ASD patients and the functional outcome or quality of life parameters; 5) the influences that certain types of pharmacological or psychotherapeutic treatments have on EF in adult ASD patients.
The current article focuses on the first of the above mentioned directions, namely on the performance of adults with ASD with a normal intelligence quotient, compared to adults with no psychiatric pathology, during specific tasks of executive functions (inhibition, cognitive flexibility, planning, working memory, generativity) (48 articles out of a total of 71). (Table 1).
INHIBITION
Inhibition, the executive function defined as the ability to reject an automatic tendency in a specific situation, has been studied in relation to psychiatric pathology, with most studies focusing on ADHD. In the current review, a total of 19 studies was identified that have reported the performance level of adults with ASD compared to healthy subjects during tasks involving inhibitory control. Considering the high variability of the obtained results, which can be at least partially attributed to the variety of methods used in these studies (see Table 1), we chose to review this research, depending on the type of task/ test that was used, a direction which will subsequently be followed in the presentation of the results of other executive functions.
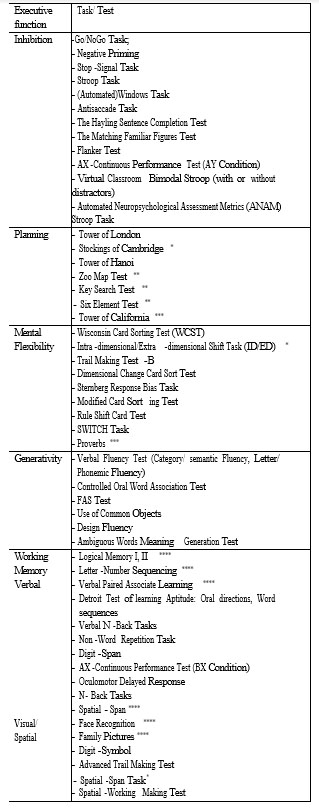
Table 1: Executive function tasks used in the studies of adults with ASD without Intelectual Disability
* Cambridge Neuropsychological Test Automated Battery
(CANTAB)
** Behavioural Assessment of the Dysexecutive Syndrome
(BADS)
*** Delis-Kaplan Executive Function System (D-KEFS)
**** Wechsler Memory Scale- Revised (WMS-R)
Most studies using the Stroop test or its variants for evaluating inhibitory control reported no significant differences between adults with ASD and healthy subjects. Thus, Ambery et al in 2006, comparing 27 adults with Asperger’s Syndrome with 20 healthy subjects, all with a Verbal Intelligence Coefficient (VIQ) greater than 80, found no significant difference between groups on the Stroop test interference score (8). Lopez et al., In 2005, used a variant of the Stroop Test (The California Stoop Test) without finding any differences between groups in any of the test’s parameters (Word-reading, Color-naming, Interference, Switching) (9). Similar results for Stroop type tasks were also obtained by Schmitz’s 2006 research, by Hill in 2006, and by Johnston in 2011, with no significant differences between groups–(1012). Two other studies have obtained mixed results, varying either between the tasks used (13) or between the parameters obtained in the same test (14). Thus, Sachse et al. showed that adult subjects with high-functioning autism performed Stroop test tasks in a significantly longer time than healthy subjects, but the overall Interference Stroop score did not vary significantly between groups (14). Parsons et al. have evaluated the inhibitory response through four Stroop-type tasks, namely a classic paper version (from the D-KEFS battery), a computerised variant (“Automated Neuropsychological Assessment Metrics Stroop task”) and two Stroop tasks using virtual reality techniques, with and without external distractors (“Virtual Classroom Bimodal Stroop with or without distractors”) (13). Thus, no significant differences were obtained between ASD and healthy adults in the classical Stroop test, the computerized or virtual reality without external distractors test, but people with ASD had significantly weaker results using virtual reality with external, visual and hearing distractors(13).
Another type of tasks commonly used in studies that looked at inhibitory control are the “Go / no-Go” tasks, but the results, as with studies using Stroop tasks, are varied, indicating both the existence of deficits among adults with ASD – (1517) and the lack thereof (10,16,18,19). Lai et al., in a comparative study on 64 adults with ASD (32 men and 32 women) and 64 healthy adults (32 men and 32 women) found a significant effect of the disorder on the results of a Go / no-Go task and the lack of a relevant effect of the gender (15). In 2014 Wilson used this task to compare the inhibitory response between
89 adults with ASD and 89 healthy adults, and, after adjustments based on the Performance Intelligence Quotient (PIQ), found significant differences between groups with significantly more mistakes made by the ASD group both at the Go (omissions) and the no-GO (commissions) conditions, but with no differences in the Beta coefficient (measure of response biases) (17). In an attempt to determine a possible relationship between the type of response to inhibitory tasks and the general arousal state of adults with ASD, Raymakers et al. emphasize the presence of a general state of overarousal in ASD individuals. The authors of this study justify this conclusion by describing an inhibition deficiency in a Go/ no-Go task with a fast presentation rate (1 second) and a lack of deficit compared to healthy subjects when the presentation of the stimuli takes place at 2 and 6 seconds respectively (16). Two functional neuroimaging studies, which included in the analysis the evaluation of emotional stimuli (happy and sad faces) inhibition through Go/ no- Go tasks, found no significant difference in performance between the two groups groups (18,19). As the a f o r e m e n t i o n e d s t u d i e s f o c u s e d o n f u n c t i o n a l neuroimaging, the studied groups were very small (approximately 15 participants per group) and thus, although in Duerden’s study the ASD subjects made a noticeable number of errors, the difference was not significant.
The “Hayling Sentence Completion Test” is an instrument through which the initiation of the response and the inhibitory control are measured. Thus, the first part of the test involves the completion of certain sentences (initiation of the response) by the subject being evaluated, and the second part also involves the termination of sentences, but with a word that is inappropriate to the meaning of the sentence (inhibition of an appropriate response). In 2011, Johnston, after using this test in a comparative study on adult subjects with ASD, ADHD, and healthy adults respectively, reported that adults with ASD were significantly slower in inhibiting the prepotent response and in completing section 1 of the test, related to response initiation (12). Another study, conducted by Hill and his collaborators in 2006 showed that although there are significant differences between ASD adults and healthy subjects concerning the time needed for the completion of each of the two sections but also in terms of the overall score, there is the possibility that these differences are at least partially explained by the difficulty of initiating a response, given that once the analysis is adjusted according to the duration obtained for the first part of the test, there was no longer a significant difference in the latency of response during the second section of the test (the task that involves inhibition) (11). Additionally, in Hill’s study, the total number of errors made in this test did not differ significantly between the studied groups, whilst another study using the Hayling test found that subjects with ASD make significantly more errors than healthy ones (11,20). No differences between groups on the Hayling test were also reported by Baez in 2012(21).
Another test that has shown the existence of inhibitory function deficits in adults with ASD is the “Antisaccade” task, in which the evaluated person, looking at a static target, has to look at the opposite side of the one in which a certain luminous stimulus appears (22,23). Tasks related to inhibitory control that showed a similar level of performance between adults with ASD and healthy controls are the Flanker Test (21), the “Matching Familiar Figures” test (12), and “AX-Continous Performance Test” (24) – a two-condition test, one investigating the inhibitory function and the second – working memory.
MENTAL FLEXIBILITY
Mental (cognitive) flexibility defines an individual’s ability to continuously adapt their cognitive strategies to deal with new environmental stimuli. This cognitive function is often divided into two components – a conscious, voluntary one used to shift from one task to the other (“cognitive shifting”) and an involuntary, unconscious component involved in fast adaptation to new tasks/situations (“task switching”). The lack of cognitive flexibility has often been incriminated as the pathophysiological basis of certain symptoms specific to the autistic spectrum, such as repetitive interests and behaviours, adherence to routines, and so on. Of the studies included in this review, 22 compared the performance of adult subjects with ASD versus healthy subjects. Tasks that tested cognitive flexibility varied (see Table 1), the most commonly used being variants of the “Wisconsin Card Sorting Test” and the “Trail Making Test”.
The “Wisconsin Card Sorting Test” (WCST) is the most commonly used task in neuropsychology studies for the evaluation of cognitive flexibility and from its introduction in the mid-20th century until today, a number of modified versions have emerged. In the classic version, the test includes 4 key cards and 128 response cards, all with geometric figures, cards varying in shape, colour or number of geometric figures. The person being evaluated must discover the rule by which the cards are sorted (number, colour or shape) through successive attempts, each followed by the evaluator’s feedback. Once the rule is discovered, the evaluated person must keep sorting the cards, according to the discovered principle. After 10 consecutive correct answers, the rule changes without notifying the evaluated person. The test’s parameters, used as dependent variables vary and among them there are: the total number of errors, the number/ percentage of perseverative errors (the Milner type – incorrect response immediately after a completed category; the Nelson type – incorrect response after another incorrect response in the same category), the number/ percentage of non- perseverative errors, the number of completed categories, conceptual-level responses, etc. The variability of the results reported by studies that investigated cognitive flexibility in adult patients with ASD is quite large, but equally unstable is the choice of test parameters considered to be of interest in these studies. Keeping this observation in mind, it is difficult to consider the results of these research papers as significant or not in terms of differences between adults with ASD and those included in control groups.
Sumiyoshi et al, in 2011, after comparing 22 adults with ASD and 15 healthy adults, described the presence of some cognitive flexibility deficits among adults with ASD in solving the WCST test, as these subjects made significantly more perseverative errors and completed significantly fewer categories, but had a similar reaction time (25). Significant differences were also reported by Broden in 2017, Lopez in 2005, and Rumsey et al. in their studies, the latter using a larger number of test parameters (including non-perseverative errors, conceptual-level responses) – (9,2629). On the other hand, a number of studies could not identify significant differences between ASD and healthy adults (30), or the results were mixed –(8,3133). Thus, in 2014, Yasuda, after comparing 33 adults with ASD with 33 healthy subjects matched by age, sex, education, and intelligence quotient, showed that adults with ASD make a significantly greater number of errors in the WCST test, including Milner or Nelson type perseverative errors, but the total number of completed categories did not differ significantly(33). A study performed by Kiep in 2017 in an attempt to compare cognitive flexibility performances between genders, compared 99 men with ASD and 40 women with ASD, with 35 men and 25 women respectively without a psychiatric disorder (32). The results obtained in the WCST test showed that both male and female adults with ASD made significantly more perseverative errors, whereas the number of non- perseverative errors and the number of completed categories were significantly different only among male participants(32).
“The Modified Card Sorting Test” is one of the revised versions of WCST, that has common principles, but with a few differences. Thus, there are fewer cards (only 48 compared to 128), only 6 consecutive correct responses are required to consider a category as complete, and the changing of the sorting rule is announced by the evaluator. The MCST test was also used in studies with adult subjects with ASD, but all studies reported no significant differences between ASD and healthy subjects (11,34,35). The study done by Geurts et al. is one of the few studies that have been based on older adult populations, as the authors compared 23 subjects with ASD with an equal number of healthy subjects, all aged between 51 and 83, but there were no statistically significant differences in the number of perseverative errors made by them in the MCST test(34).
“The Trail Making Test” (TMT) is a tool that measures processing speed together with cognitive flexibility. Both activities included in the test involve drawing lines, with the purpose of connecting 25 circles randomly placed on an A4 page. In Part A of the test, which measures the cognitive processing speed, the circles contain numbers from 1 to 25, and in part B of the test, they contain letters from A to L and numbers from 1 to 13 and the person being evaluated must connect letters and numbers alternately (“A-1-B-2-C […]”). For both parts, the time needed to complete the task is noted, and for the cognitive flexibility evaluation, the most frequently used measures are the TMT-B (execution time of part B) and the ‘TMT B – TMT A’ variable. Most studies using the TMT test for assessing cognitive flexibility, comparing ASD and healthy adults, did not find significant differences between groups with respect to the TMT-B variable (9,21,29,30,35,36), the TMT B – TMT A variable (34,35) or the TMT-B with the time needed to perform the first part as a covariate in the analysis (11). Two studies report significant differences between ASD and healthy adults, with a significantly longer time needed by the patients with ASD to perform the B part (37,38), but none of these studies tested the survival of a significant result after taking into account the cognitive processing speed (TMT A). Towgood et al., after finding no significant differences between groups through simple comparison, have then performed a multiple case series analysis (35). Thus, they found that the performance of ASD subjects in a number of tests, including TMT was heterogeneous, more variable than the one of healthy subjects, participants showing different weaknesses and strengths (35). In the case of the TMT test, 19% of ASD subjects had TMT B and TMT B- TMT A times above 2 standard deviations compared to the mean of the control group and 28% of ASD subjects below 2 standard deviations(35).
The absence of deficits in the cognitive flexibility of adults with ASD has also been reported in other studies using less utilised tasks than the aforementioned, such as the “Intradimensional/ extradimensional set shifting task” which is part of the CANTAB tests battery (14), “SWITCH” (10), “The Dimensional Change Card Sort” (21), “Rule Shift Card” as part of the BADS tests battery (11) or “Proverbs”, as part of the DKEFS tests battery (35). Two other studies report significant differences among the ASD and control groups, using less common tasks as well, such as a computerised version of the Sternberg test (39), or even an original set shifting task(40).
PLANNING
Planning is an executive function characterized by formulating, analyzing and selecting ideas and actions in order to achieve a particular goal. As it is the case with other executive functions, planning is evaluated through a multitude of tests (Table 1) with a wide range of variables used. The most well-known tests are the “Tower of London” and “Tower of Hanoi”, and these have numerous adaptations.
All the “Tower of London” (ToL) tasks use the same general principle, which involves the arranging of three balls/ beads on three pegs, according to a pre-set configuration shown by the evaluator. Variations of ToL differ by the number of configurations that the person has to perform (e.g. 10, 20, etc.), the difficulty of the task quantified by the number of moves required to make the configurations (there are tasks using configurations with
1-3 moves, with 3-6 moves, 4-7 moves, etc.), by the presentation of the task (physical/ on-screen) and by the parameters used as dependent variables. Of the studies included in this review, three using ToL tasks have been identified (34,41,42). Two of these studies include groups of older adults with ASD (the mean age of adults with ASD in the Davids et al. study is 58.6 and in Geurts’s study of 63.6), and all three studies fail to find significant differences in performance between ASD and healthy subjects in most comparisons of ToL variables (34,41,42). The only mixed result was obtained by Davids et al. in their study, reporting the lack of differences in the score that quantifies the number of correctly solved configurations and significant differences between groups on the total planning and execution time (41). Another study by Sachse et al. in 2013 used a ToL-derived computerized task called “Stocking of Cambridge”, and the results of the analysis also show the lack of significant differences between groups(14).
The “Tower of Hanoi” (ToH) tasks, similar to ToL, imply the existence of 3 bars as well, but in this case, usually on the left bar, a number of disks (e.g. 3, 5, etc) are placed by size, from the biggest disc at the bottom, to the smallest at the top. The goal of the test is to move all of the disks to get the same configuration on another bar, usually the one on the right, taking into account a few rules. Two studies used ToH tasks to compare the planning ability of adults with ASD and of healthy individuals and the results showed no differences between groups (32,36). In the study of Kiep et al. from 2017 the differences were analyzed considering gender, and the results were that men and women with ASD had similar performances to those of neurotypical adults (32). Lopez et al., in 2005, used a modified version of ToH called “Tower of California” and found that adults with ASD built fewer towers than those in the control group(9).
In addition to these classical tasks that investigate the cognitive planning ability, some researchers have attempted to use tests that involve solving tasks that are closer to those encountered in real life. Tasks such as “Zoo Map” and “Key Search”, both of which are part of the “Behavioral Assessment of the Dysexecutive Syndrome” (BADS) battery, evaluate people’s ability to plan a route on a map taking into account certain rules and, respectively, to find a lost object in a certain area drawn on paper.
The “Zoo Map” task is quite commonly used in more recent studies and the search for routes on the map is done in two separate sections. In the first section the route must be planned by the evaluated individual, but in the second more structured section, the participant only has to follow certain predefined routes. For both parts, the planning time, the actual execution time, the total time, and an accuracy score in solving the problem are noted. Variables most commonly used as indicators of planning ability are those resulted from the first part of the test or indicators resulting by subtracting the results of part 2 of the test from part 1. Five studies used this task among adult subjects with ASD, with no differences between them and healthy subjects reported in three of those (20,35,41). Two other studies, however, report a longer planning and execution time for subjects with ASD (43), lower accuracy (11), longer total time (43) and a lower total score (11) (all parameters were reported for the first part of the test).
The “Key Search” task, which implies searching for a lost object in a drawn area, requires, besides the ability to plan, the ability to form strategy and to solve a problem. In 2006 Hill used this task and, using a scoring procedure that quantifies how efficiently the participant covered the area investigated, did not find significant differences the performance of adults with ASD compared to a group of healthy subjects (11). Bramham in 2009, using the same task, concluded that the 45 subjects with ASD spent significantly more time in solving the task than the 35 neurotypical subjects, but the accuracy in solving the task did not differ between groups(43).
WORKING MEMORY
Working memory represents a complex cognitive function involved in the temporary storage and processing of information so that it can be manipulated later on in different mental tasks or goal-oriented behaviours. According to Baddeley’s model, working memory can be divided into three components – the executive centre (which selects information, processes it and directs it to one of the following components), the phonological loop and the visuospatial sketchpad (44). Thus, the tasks that test working memory, access one of the two components of the working memory – the verbal (phonological) or the visuospatial memory, by the nature of the information needed to be manipulated.
The most common type of tasks in studies that investigate working memory in general, but also in the studies included in this review, are the “N-back” tasks. Although there is a wide variation between the different N-back tasks, the general principles remain the same. Thus, the evaluated person has to correctly identify a certain target (e.g. letter, drawing, human face, etc.), according to the instructions received from the evaluator, from a simple identification (“Press the button when the letter F appears”, O-back) to the identification of the target when it appears twice in a row (1-back) and respectively when the target is represented by a stimulus identical to the one presented two positions behind (2-back). The most commonly reported parameters in the studies are: the reaction/ response time, the number of correct answers/ accuracy and the number of errors (by omitting the target or by pressing false targets). Seven studies included in this review used N-back tasks to compare working memory between adult subjects with ASD and healthy controls. Of these, five used tasks for the verbal working memory (tasks with letters or numbers) –(21,26,4547) and two used as tasks visual targets like simple drawings (48) or human faces (49). All studies that analysed N-back tasks with letters or numbers showed the lack of significant differences between groups in terms of reaction/ response times – (21,26,4547), of errors (46,47), or of correct answers/ accuracy (21,26). Koshino et al in 2008, who used a N-back task with human faces in their functional neuroimaging study, found no significant differences between the groups for the reaction time or the number of errors made during a task (49). The study done by Lever et al. in 2015, distinguished by the large number of subjects (111 participants with ASD and 164 neurotypicals), used a N-back task with black and white drawings of common objects, thus accessing the visual component of the working memory (48). The results of this study show the presence of significant differences in terms of reaction time between participants with ASD and healthy controls, but with a similar number of correct answers(48).
In addition to N-back tasks, a large number of other tests have been identified that evaluate the verbal (9 tasks) and visuospatial (8 tasks) working memory (Table 1). We will summarize, for each task, the number of studies which reported significant differences between the groups, the subjects with ASD being less well-performing than the healthy ones (“diff sig.”), the number of studies without significant differences (“no diff.”) and the number of studies with mixed results (“mix”), when within the same task there are significant differences between groups in some test variables, but not in all of them.
Thus, the tasks used for the evaluation of the verbal working memory were: “Logical Memory I” (diff. sig.- 1 study(28) , no diff.- 2 studies(8,50)), “Verbal Paired Associates Learning” (no diff.- 2 studies (8,50)), “Non- word Repetition Task” (no diff.- 1 study (15)), “Span- Counting Task” (no diff. – 1 study (21)), “AX Continous Performance Test” BX Task (diff. sig. – 1 study (24)), “Letter – Number Sequencing” (diff. sig. – 2 studies (32,51), no diff.- 3 studies (9,45,50)), “Oral Direction” and “Word Repetition” as part of the “Detroit Test of Learning Aprtitude-2” battery of tests (diff. sig. – 1 study(52)).
The following results were reported after the evaluation of the visuospatial component of the working memory: “Spatial Span” (diff. sig.- 3 studies (34,45,50)), “Oculomotor Delayed Response” (mix – 2 studies (23,53)), “Spatial Working Memory” (no diff. – 1 study (14), but with large effect size), “Face Recognition” (diff. sig.– 1 study (50)), “Family pictures” (diff. sig.– 1 study (50)), “Advanced Trail Making Test” (no diff.– 1 study (54)), “Digit symbol” (diff. sig.– 1 study (54)), “Design Memory I” (diff. sig.– 1 study(28)).
GENERATIVITY
G e n e r a t i v i t y r e p r e s e n t s t h e a b i l i t y t o spontaneously generate new ideas and behaviors. A deficient individual in this executive area lacks spontaneity and initiative, may have poor speech and a reduced capacity to engage in imagination games.
The studies included in this review used certain phonemic and semantic verbal fluency tasks, but also design (drawing) tasks to test generativity among adult persons with various forms of autism. As with the other executive functions studied and synthesized in this analysis, the results reported in the 15 articles that used either phonemic or phonemic and semantic combined tasks for verbal fluency testing, are variable.
The most commonly used verbal fluency task is the phonemic fluency task (with letters/ phonological) – Controlled Oral Word Association Test (COWAT), which requires a series of words to be generated, starting from a certain letter, most often F, A or S, but also adaptations done according to the native language of the participants, within a fixed amount of time, usually one minute. The number of correct words produced is recorded as a parameter.
For the phonemic fluency tested by COWAT or its variants, 6 studies (8,28,34,38,43,55) identified a significant difference between the number of words generated by the autism group and the control group with fewer correct words produced by autistic participants, while 8 studies (9,11,15,17,21,30,35,41) did not find a significant difference between the number of words generated by autistic subjects compared to the healthy ones. One final article, which included 139 ASD participants: 99 men and 40 women versus 60 healthy subjects: 35 men and 25 women, reported mixed results depending on the gender of the participants (32). Thus, in the phonemic fluency test using the letters “K” and “M” (adapted to the native Dutch language), significant differences from the control groups were recorded only for men with autism, not for women, and only for the letter “K”, not for the letter “M”(32).
In addition to phonemic fluency, within generativity, the semantic (or category) fluency is tested as well, for which the participant is asked to produce as many words as possible from a certain category, most often animals, but also others such as crafts, fruits and vegetables, vehicles and others. The number of correct words generated for a category in a certain time limit is tested in this case as well.
Semantic fluency was tested in the same study by Kiep in 2016 using two categories: animals and professions, with mixed results depending on gender. Significant differences were identified for men with autism but not for women (32). Other 5 studies included semantic fluency in the analysis, two of which identified a significant difference between autistic and healthy subjects (38,55), two did not report statistically significant differences between groups (9,41), while Minshew et al. in 1992 obtained statistical significance only before applying the Bonferroni correction(30).
Non-verbal or design fluency is another parameter used to assess generativity, and a task commonly used in its assessment involves three types of drawing patterns, two simple designs and one involving switching designs. Two studies that reported results on this test have been identified, but the findings were contradictory (9,21). Thus, Lopez in 2005 found significant differences in the number of perseverations – more in the group of autistic persons, for simple designs (9), while Baez in 2012 reported significant differences between groups only for the switching task(21). CONCLUSIONS
This review of the studies, which compared the results of adults with ASD and intact cognitive ability on performance-based tasks of executive functions with the performance of neurotypicals, brings forth a considerable variability of the results together with a lack of consensus regarding the patterns of deficits of executive functions in this clinical population. Out of all the studies reviewed, we found no executive function or even part of one to yield conclusive results in either direction. Furthermore, some studies draw attention to the heterogeneity of these deficits even when considering relatively homogeneous populations, with similar severity of the disorder and level of intelligence, age and levels of education. This considering, it is our opinion that in order to draw pertinent conclusions regarding executive function deficits in autism, it is imperative to employ the same neurocognitive test battery in studies, replicated several times, on large numbers of participants.
Moreover, there are other sources of uncertainty besides differences in performance on executive function tests, such as variability in the activation and connectivity of different cerebral areas as found in neuroimaging studies using EF tasks, or the relationship between EF patterns and autistic core symptoms, other clinical traits or even general level of functioning in daily life.
Finally, it is our belief that a more extensive knowledge of the weak and strong points in executive functioning of persons with ASD could provide valuable information with regards to individualized therapeutic interventions, tailored to each person’s needs (e.g. therapeutic measures to increase cognitive flexibility or working memory, pharmacological interventions to lower the overarousal for better inhibitory control, etc).
REFFERENCES:
1.Goldstein S, Naglieri JA. Handbook of executive functioning. Handb Exec Funct 2014;1–567.
2.Russo N, Flanagan T, Iarocci G, Berringer D, David P, Burack JA. Deconstructing executive deficits among persons with autism : Implications for cognitive neuroscience. Brain and cognition 2007;65:77–86.
3.Brambilla P, Hardan AY, Ucelli di Nemi S, et al. The Functional Neuroanatomy of autism. Funct Neurol 2004;19(1):9–17.
4.Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review 2004;24.
5.Hill EL. Executive dysfunction in autism. Trends Cogn Sci 2004;8(1):26–32.
6.Kenworthy L, Yerys BE, Anthony LG, Wallace L. Understanding executive control in autism
spectrum disorders in the lab and in the real world. 2010;18(4):320–38.
7.O’Hearn K, Asato M, Ordaz S, Luna B. Neurodevelopment and executive function in autism. Dev Psychopathol 2008;20(4):1103–32.
8.Ambery FZ, Russell AJ, Perry K, Morris R, Murphy DGM. Neuropsychological functioning in adults with Asperger syndrome. Autism 2006;10(6):551–64.
9.Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord 2005;35(4):445–60.
10.Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DGM. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry 2006;59(1):7–16.
11.Hill EL, Bird CM. Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia 2006;44(14):2822–35.
12.Johnston K, Madden AK, Bramham J, Russell AJ. Response inhibition in adults with autism spectrum disorder compared to attention deficit/hyperactivity disorder. J Autism Dev Disord 2011;41(7):903–12.
13.Parsons TD, Carlew AR. Bimodal Virtual Reality Stroop for Assessing Distractor Inhibition in Autism Spectrum Disorders. J Autism Dev Disord Springer US; 2016;46(4):1255–67.
14.Sachse M, Schlitt S, Hainz D, et al. Executive and visuo-motor function in adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2013;43(5):1222–35.
15.Lai MC, Lombardo M V., Ruigrok ANV, et al. Cognition in Males and Females with Autism: Similarities and Differences. PLoS One 2012;7(10).
16.Raymaekers R, van der Meere J, Roeyers H. Event-Rate Manipulation and its Effect on Arousal Modulation and Response Inhibition in Adults With High Functioning Autism. J Clin Exp Neuropsychol (Neuropsychology, Dev Cogn Sect A) 2004;26(1):74–82.
17.Wilson CE, Happé F, Wheelwright SJ, et al. The Neuropsychology of Male Adults With High-Functioning Autism or Asperger Syndrome. Autism Res 2014;7(5):568–81.
18.Duerden EG, Taylor MJ, Soorya L V., Wang T, Fan J, Anagnostou E. Neural correlates of inhibition of socially relevant stimuli in adults with autism spectrum disorder. Brain Res 2013;1533:80–90.
19.Shafritz KM, Bregman JD, Ikuta T, Szeszko PR. Neural system mediating decision-making and response inhibition for social and non social stimuli in autism. Prog Neuropsychopharmacol Biol Psychiatry
2015;3(60):112–20.
20.Boucher J, Cowell P, Howard M, et al. A combined clinical, neuropsychological, and neuroanatomical study of adults with high functioning autism. Cogn Neuropsychiatry 2005;10(3):165–213.
21.Baez S, Rattazzi A, Gonzalez-Gadea ML, et al. Integrating intention and context: assessing social cognition in adults with Asperger syndrome. Front Hum Neurosci 2012;6(November):1–21.
22.Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology
1999;52(5):917–917.
23.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of Executive Function in Autism. Biol Psychiatry 2007;61(4):474–81.
24.Bodner KE, Beversdorf DQ, Saklayen SS, Christ SE. Noradrenergic moderation of working memory impairments in adults with autism spectrum disorder. J Int Neuropsychol Soc 2012;18(3):556–64.
25.Sumiyoshi C, Kawakubo Y, Suga M, Sumiyoshi T, Kasai K. Impaired ability to organize information in individuals with autism spectrum disorders and their siblings. Neurosci Res; 2011;69(3):252–7.
26.Braden BB, Smith CJ, Thompson A, et al. Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Res 2017;10(12):1945–59.
27.Rumsey JM. Conceptual problem-solving in highly verbal, nonretarded autistic men. J Autism Dev Disord 1985;15(1):23–36.
28.Rumsey JM, Hamburger SD. Neuropsychological divergence of high-level autism and severe dyslexia. J Autism Dev Disord
1990;20(2):155–68.
29.Rumsey JM, Hamburger SD. Neuropsychological Findings in High- Functioning Men with Infantile Autism, Residual State.
J Clin Exp Neuropsychol 1988;10(2):201–21.
3 0 . M i n s h e w N J , M u e n z L R , G o l d s t e i n G , P a y t o n J B . Neuropsychological functioning in nonmentally retarded autistic individuals. J Clin Exp Neuropsychol 1992;14(5):749–61.
31.Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage 2007. p. 1219–30.
32.Kiep M, Spek AA. Executive functioning in men and women with an autism spectrum disorder. Autism Res 2017;10(5):940–8.
33.Yasuda Y. Cognitive inflexibility in Japanese adolescents and adults with autism spectrum disorders. World J Psychiatry 2014;4(2):42.
34.Geurts HM, Vissers ME. Elderly with Autism : Executive Functions and Memory. J Autism Dev Disord 2012;42: 665–75.
35.Towgood KJ, Meuwese JDI, Gilbert SJ, Turner MS, Burgess PW. Advantages of the multiple case series approach to the study of cognitive d e f i c i t s i n a u t i s m s p e c t r u m d i s o r d e r. N e u ro p s y c h o l o g i a
2009;47(13):2981–8.
36.Losh M, Adolphs R, Poe MD, et al. The Neuropsychological Profile of Autism and The Broad Autism Phenotype. North 2009;66(5):518–26.
37.Powell PS, Klinger LG, Klinger MR. Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. J Autism Dev Disord 2017;47(10):3204–19.
38.Kasirer A, Mashal N. Verbal creativity in autism: comprehension and generation of metaphoric language in high-functioning autism spectrum d i s o r d e r a n d t y p i c a l d e v e l o p m e n t . F ro n t H u m N e u ro s c i 2014;8(August):1–8.
39.Bogte H, Flamma B, Van Der Meere J, Van Engeland H. Cognitive flexibility in adults with high functioning autism. J Clin Exp Neuropsychol 2008;30(1):33–41.
40.Shafritz KM, Dichter GS, Baranek GT, Belger A. The Neural Circuitry Mediating Shifts in Behavioral Response and Cognitive Set in Autism. Biol Psychiatry 2008;63(10):974–80.
41.Davids RCD, Groen Y, Berg IJ, Tucha OM, van Balkom IDC. Executive Functions in Older Adults With Autism Spectrum Disorder: Objective Performance and Subjective Complaints. J Autism Dev Disord 2016;46(9):2859–73.
42.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fmri study of an executive function task and corpus callosum morphometry. Cereb Cortex 2007;17(4):951–61.
43.Bramham J, Ambery F, Young S, et al. Executive functioning differences between adults with attention deficit hyperactivity disorder and autistic spectrum disorder in initiation, planning and strategy formation. Autism 2009;13(3):245–64.
44.Baddeley A, Hitch G. Working memory. Psychol Learn Motiv 1974;8:47–89.
45.Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. J Autism Dev Disord 2005;35(6):747–56.
46.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage 2005;24(3):810–21.
47.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biol Psychiatry 2007;62(3):198–206.
48.Lever AG, Werkle-Bergner M, Brandmaier AM, Ridderinkhof KR, Geurts HM. Atypical working memory decline across the adult lifespan in autism spectrum disorder? J Abnorm Psychol 2015;124(4):1014–26.
49.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NK, Just MA. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb Cortex 2008; 18(2): 289–300
50.Williams DL, Goldstein G, Minshew NJ. Impaired memory for faces and social scenes in autism: Clinical implications of memory dysfunction. Arch Clin Neuropsychol 2005;20(1):1–15.
51.Zimmerman DL, Ownsworth T, O’Donovan A, Roberts J, Gullo MJ. Independence of Hot and Cold Executive Function Deficits in High- Functioning Adults with Autism Spectrum Disorder. Front Hum Neurosci 2016;10:1–14.
52.Minshew NJ, Goldstein G, Siegel DJ. Speech and language in high functioning autistic individuals. Neuropsychology 1995;9(2):255–61.
53.Luna B, Minshew NJ, Garver KE, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology 2002;59:834–40.
54.Nakahachi T, Iwase M, Takahashi H, et al. Discrepancy of performance among working memory-related tasks in autism spectrum disorders was caused by task characteristics, apart from working memory, which could interfere with task execution. Psychiatry Clin Neurosci 2006;60(3):312–8.
55.Kleinhans NM, Müller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res 2008;1221:115–25
***



