ELEVATED PLASMA FIBRINOGEN A POSSIBLE BIOMARKER FOR PSYCHOLOGICAL DISTRESS AND DEPRESSION
Abstract
Depressive disorder represents the leading cause of disability worldwide. An increasing body of evidence suggests that patients presenting psychological distress and depression show alterations in immunological markers. The aim of our study was to observe the correlation of fibrinogen as a potential biological marker for psychological distress and depression. Data analyzed in this paper refers to patients (N=100) included in a prospective study conducted between 01 February-30 June 2016, males and females, age>18 years, recruited from the patients whom presented for diverse para-clinical evaluations in the outpatient clinic National Institute of Gerontology and Geriatrics “Ana Aslan” (NIGG “Ana Aslan”), Bucharest, Romania. Socio-demographic information collected included: gender, age, marital status, environmental origin, level of education, socio- economic status. For all the participants of the study fibrinogen was measured using a turbidimetry method. Psychological distress and depressive symptoms were evaluated using the Hospital Anxiety and Depression Scale (HADS). From the total number of patients 64% were female, with a mean age of 67 years, from the urban area (92%), retired (80%).We found a mean value of fibrinogen of 301.16 mg/dL (SD ± 63.20). At HADS-global the patients presented a mean value of 15.9 (SD ± 7.31), while at the HADS-D the mean value was 6.41 (SD ± 3.60). Low statistically significant positive correlations between fibrinogen and HADS-global ( rs(98)= .30, p= 0.0023) and HADS-D (rs(98)=.36, p=0.0003) were found. Our study results were consistent with the data literature reported from previous studies showing a significant positive correlation between psychological distress, depression and fibrinogen. Also, we found higher levels of plasma fibrinogen when we compared patients presenting psychological distress with those without psychological distress, even though the effect size was rather small.
Introduction
Depression is a pervasive, complex and heterogeneous disorder and represents the leading cause of disability worldwide in terms of total years lost due to disability (1). Current neurobiological theories are based on studies investigating psychosocial stress and stress hormones, neurotransmitters such as serotonin, norepinephrine, dopamine, glutamate and gamma-aminobutyric acid (GABA), altered neuro-circuitry function, altered hypothalamic-pituitary-adrenal axis activity, neurotrophic factors, and circadian rhythms (2). Several hypotheses concerning the biologically based cause of depression have been suggested over the years, including theories revolving around monoamine neurotransmitters, neuroplasticity, inflammation and the circadian rhythm (3, 4, 5). Immunological mechanisms have been implicated in the complex pathophysiology of depression providing leads for further investigation of the hypothesis considering that inflammation represents an important etiological factor of depression. Despite the fact that the exact disease mechanism is unknown, some previous studies have shown that low-grade inflammation possibly plays a role in the development of depression by indicating an association between depression and elevated inflammatory markers such as cytokines and acute phase proteins (6, 7, 8). Also growing evidence indicates that psychological distress is accompanied by activation of the innate immune system, resulting in increased monocyte production of the pro-inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α and upregulation of the acute phase response with increased synthesis of fibrinogen and C-reactive protein (9). Fibrinogen exerts pro-coagulant function as a precursor of fibrin in the coagulation cascade and as cofactor for platelet aggregation and also a major acute phase reactant synthesized by the liver during inflammation (10). Jensen et al. showed that fibrinogen stimulates synthesis of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α from peripheral blood mononuclear cells, and hereby to increase levels of pro-inflammatory cytokines (11). These pro-inflammatory cytokines may activate the enzyme indolamine-2,3-dioxygenase, which degrades the precursor of serotonin, tryptophan (11). The decreased concentrations of tryptophan determine a reduced serotonin synthesis, which may lead to the development of depression (12, 13). As fibrinogen is the precursor of fibrin for production of thrombi, another possible hypothesis could be that increased fibrinogen levels could lead to increased risk of thrombus formation in vessels in the brain, that secondary may lead to psychological distress and depression. On the other hand another possibility is that psychological distress and depression could lead to increased fibrinogen levels. In several studies increased levels of depressive symptoms, and clinical depression, respectively, have been associated with elevated fibrinogen (6, 14, 15, 16), although others failed to find a significant association (17-21).
The aim of our study was to analyze if there is a correlation between the inflammatory markers like fibrinogen and depressive symptoms and if fibrinogen could be a potential biological marker for psychological distress and depression symptoms severity.
Material and methods
Data for present paper was obtained after an analysis of a prospective study conducted between 01 February – 30 June 2016, on a sample comprised 101 adult participants, males and females, aged over 18 years, recruited from the patients whom presented for various para-clinical evaluations in the outpatient clinic National Institute of Gerontology and Geriatrics “Ana Aslan” (NIGG “Ana Aslan”), Bucharest, Romania. Details of the study were explained to every patient and a written informed consent was obtained from all participants. The socio- demographic data collected included: gender, age, marital status, environmental origin, level of education, socio- economic status. Data about family history, pathological personal history, diagnosis of depressive disorder, symptoms at onset, number of hospitalizations for diagnosis of depressive disorder, previous antidepressant treatment, association with psychotropic medication, quality of response and side effects of medication, and somatic comorbidities were also collected. For all patients were recorded the following laboratory results: CBC, GGT, glucose, total cholesterol, triglycerides, TSH, fibrinogen, VSH, CRP and CRP-hs. All the participants required to complete the Hospital Anxiety and Depression Scale (HADS). HADS was designed to rate the symptom level of depression (HADS-D) and anxiety (HADS-A) and emotional distress in non- psychiatric populations (22). There are two subscales, each of which has seven items to rate depression (HADS-D) and anxiety (HADS- A) on a 4-point Likert scale (0 = not at all, 3 = mostly; range = 0–21 points). The recommended cut-off of ≥ 8 points to identify cases of elevated levels of anxiety and depression symptoms had a sensivity and specificity of both subscales consistently in the range of 0.70 to 0.90 (23). The approval for the HADS scale used was obtained. This study was approved by the I.O.S.U.D. U.M.F. Craiova ethical committee. We also had a collaboration protocol with the outpatient clinic NIGG “Ana Aslan”. For all participants in the study fibrinogen was measured using a turbidimetry method (Coa-DATA 2001- a 2 channel coagulation analyzer from Dutch Diagnostics). All measurements were done by laboratory technicians. The normal fibrinogen range is considered to be 200 to 400 mg/dL, although normal value ranges may vary slightly among different laboratories. Values above 400mg/dL were considered as a marker of inflammation. In the final analysis entered only 100 participants because the scale for one patient got lost.
Statistical analysis
All the statistical analysis was performed with SPSS version 21 (SPSS Inc., Chicago Ill.) and included the following tools: descriptive analysis, as the data were skewed, not normally distributed, we needed to use the Spearman correlation coefficient as a measure of the correlation and Mann-Whitney U to determine effect size. The correlation analyses express the strength of linkage or co-occurrence between to variables in a single value between -1 and +1. This value is called the correlation coefficient. A correlation coefficient of 0 indicates that no relationship between the variables exists at all. The correlation coefficient “rule of thumb” interpretation is the following: 0.90 to 1 (-0.90 to -1)= very high positive (negative) correlation; 0.70 to 0.90 (-0.70 to -0.90)= high positive (negative) correlation; 0.50 to 0.70 (-0.50 to –
0.70)= moderate positive (negative) correlation; 0.30 to
0.50 (-0.30 to -0.50 = low positive (negative) correlation;
0 to 0.30 (0 to -0.30)= negligible correlation (24). A positive correlation coefficient indicates a positive relationship between two variables (the larger variable “A”, the larger variable “B”) while a negative correlation coefficients expresses a negative relationship (the larger variable “ A”, the smaller variable “B”) (25).
Effect size (r) was determined for each pair of groups with the formula r= Z/√N [3] (where N= is the total number of participants in a pair of groups) and we used Cohen’s effect size estimates (0.2 =’small’, 0.5 = ‘medium’ and 0.8 = ‘large’ effect size) (26).
Results
The main clinical and demographical data regarding the entire group are presented in table 1. daregardingheentire group are presented in table 1
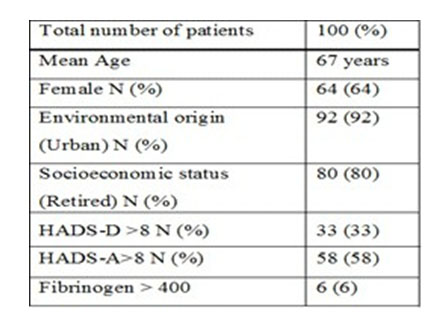
Table 1: demographic data of the study population
The mean value of fibrinogen was 301.16 mg/dL (SD ± 63.20). At the psychometric scale HADS-global the patients presented a mean value of 15.9 (SD ± 7.31), while at the HADS-D the mean value was 6.41 (SD ± 3.60). There was a low but statistically significant positive correlation between fibrinogen and psychological distress (HADS-global), rs(98)= .30, p= 0.0023 (graph 1).
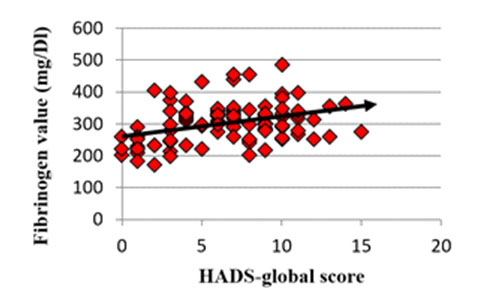
Graph 1: the statistical correlation between fibrinogen values and depression symptoms severity assed by HADS rating scale of a rs(98)= 0.30 and a p value of 0.0023. Also we found a low positive correlation, yet statistically significant between fibrinogen and depressive symptoms, HADS-D, rs(98)=.36, p=0.0003. We created two groups of patients comprising: “non-distress” group with a score HADS-global <8 and a “distress” group with a score
HADS-global ≥ 8. From the total number of 100 patients analyzed 16 (16%) were in the “non-distress” group and
84 (84%) were in the “distress” group. Patients from the “distress” group compared with patients from the “non- distress” group presented a statistically significantly higher level of fibrinogen (U= 285.00, Z= -3.640, p<0.001). However, the effect size was rather small (r=0.364).
Discussions:
In this study we analyzed the relationship between fibrinogen as a biological marker and psychological distress and depressive symptoms. Although the main limitation of this study remains the small sample size, we found a statistically significant low positive correlation between depressive symptoms and elevated plasma fibrinogen. Also we found a statistically significant low positive correlation between elevated plasma fibrinogen and psychological distress. Comparing the two groups of patients that we created (“distress” and “non-distress” group) we observed a statistically significant higher level of fibrinogen in patients with psychological distress. However, the effect size was rather small (r=0.364). Our study results are consistent with the data reported from previous studies in literature showing a significant positive association between depression and fibrinogen. As a heterogeneous disorder presenting a wide range of symptoms, the treatment success of depression varies among patients. Some studies showed that high plasma fibrinogen levels were associated with a poor antidepressant response and indicated an elevated inflammatory status present at non-responders (27). Geiser et al. found decreased fibrinogen levels in patients treated with serotonergic antidepressants compared to patients without use of serotonergic antidepressants and healthy controls (28), although others did not find that use of antidepressants in general was associated with decreased fibrinogen levels ( 16). Also, some suggest that MDD patients with increased inflammatory protein levels tend to be treatment resistant (29).
The potential clinical implication of fibrinogen as a predictive response marker in the case of antidepressant resistance would be that those specific patients could be subjected to medication that modulates fibrinogen levels before starting drug treatment to increase the likelihood of response. If further studies will confirm the putative use of fibrinogen level in resistant depressed patients it may be possible to better manage these patients by not exposing them to lengthy antidepressants trials but rather to use also other therapeutically methods.
Conclusions:
Psychological distress and depressive symptoms were statistically significant and positively correlated with elevated levels of plasma fibrinogen. Also, patients presenting psychological distress had higher levels of plasma fibrinogen compared to patients without presenting psychological distress, even though the effect size was rather small.
References:
1.Wittchen HU, Jacobi F,Rehm J et al. The size and burden of mental disorders and other disorders of the Brain in Europe 2010. Eur. Psychopharmacology, 2011; 21: 655-769
2.Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 2010;9:155-161
3.Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract, 2007; 61(12): 2030–2040
4.Palazidou E. The neurobiology of depression. British MedicalBulletin, 2012; 101: 127–145
5.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology, 2016; 16: 22-34
6.Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res, 1997; 66: 1–11
7.Howren MB, Lamkin DM, Suls J. Associations of depression with C- reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med, 2009; 71: 171–186.
8.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A metaanalysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457.
9.Black P, Garbutt L. Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research, 2002; 52, 1–23.
10.Koenig W. Fibrin(ogen) in cardiovascular disease: An update. Journal of Thrombosis and Haemostasis, 2003; 89, 601–609.
11.Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, Skjønsberg OH. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb. Haemost., 2007; 20,822—829.
1 2 . H a r o o n , E . , R a i s o n , C . L . , M i l l e r , A . H . , 2 0 1 2 . P s y c h o n e u r o i m m u n o l o g y m e e t s n e u r o p s y c h o p h a r m a c o l o g y : translational implications of the impact of inflammation on behavior. Neuropsychopharmacology Reviews 37, 137—162
13.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr. Psychiatry Rep., 2011; 13, 467—475.
14.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J et al. Inflammation and coagulation factors in persons465 years of age with symptoms of depression but without evidence of myocardial ischemia. The American Journal of Cardiology, 2002; 89, 419–424.
15.Panagiotakos DB, Pitsavos C, Chrysohoou C, Tsetsekou E, Papageorgiou C, Christodoulou G, et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. European Heart Journal, 2004; 25, 492–499.
16.Wium-Andersen MK, Orsted DD, Nordestgaard BG. Association between elevated plasma fibrinogen and psychological distress, and depression in 73 367 individuals from the general population. Mol Psychiatry, 2012; 18: 854–855.
17.Doulalas AD, Rallidis LS, Gialernios T, Moschonas DN, Kougioulis MN, Rizos I, et al. Association of depressive symptoms with coagulation factors in young healthy individuals. Atherosclerosis 186, 2006; 121—125.
18.Lahlou-Laforet K, Alhenc-Gelas M, Pornin M, Bydlowski S, Seigneur E, Benetos A, et al. Relation of depressive mood to plasminogen activator inhibitor, tissue plasminogen activator, and fibrinogen levels in patients with versus without coronary heart disease. Am. J. Cardiol., 2006; 97, 1287—1291.
19.Nabi H, Singh-Manoux A, Shipley M, Gimeno D, Marmot MG, Kivimaki M. Do psychological factors affect inflammation and incident coronary heart disease: the Whitehall II Study. Arterioscler. Thromb.Vasc. Biol., 2008; 28, 1398—1406.
20.Schroeder V, Borner U, Gutknecht S, Schmid JP, Saner H, Kohler HP. Relation of depression to various markers of coagulation and fibrinolysis in patients with and without coronary artery disease. European Journal of Cardiovascular Prevention and Rehabilitation, 2007; 14, 782–787.
21.Baune BT, Neuhauser H, Ellert U, Berger K. The role of the inflammatory markers ferritin, transferrin and fibrinogen in the relationship between major depression and cardiovascular disorders — The German Health Interview and Examination Survey. Acta Psychiatr. Scand., 2010; 121, 135—142
22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavia, 1983; 67, 361–370.
23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 2002; 52, 69–77.
24.Mukaka MM. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Medical Journal, 2012; 24(3): 69-71.
25.Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences 5th ed., 2003, Boston: Houghton Mifflin
26.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. Journal of Experimental Psychology: General, 2012; 141(1):2-18. doi: 10.1037/a0024338
27.Martins-de-Souza D, Maccarrone G, Ising M, et al. Plasma fibrinogen: now also an antidepressant response marker? Translational Psychiatry, 2014; 4(1): e352; doi:10.1038/tp.2013.129
28.Geiser F, Conrad R, Imbierowicz K, Meier C, Liedtke R, Klingmüller D, et al. Coagulation activation and fibrinolysis impairment are reduced in patients with anxiety and depression when medicated with serotonergic antidepressants. Psychiatry Clin. Neurosci., 2011; 65, 518—525.
29.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741
***



