EVOLUTION OF SYMPTOMATOLOGY AND FUNCTIONALITY OF ROMANIAN PATIENTS WITH MAJOR DEPRESSIVE EPISODE IN A COHORT OBSERVATIONAL STUDY
Abstract
Background: Depression is a common and disabling psychiatric condition which cause substantial impairment in daily functioning and increases the risk for both social and physical disability, and as a result, increases the costs for other medical services. Objectives: The study aims at underlining particularities regarding the evolution and functionality of patients with major depressive disorder (MDD) under Agomelatine treatment. The most important objectives were to evaluate the daily functionality in MDD patients under treatment, the evolution of the symptomatology and treatment adherence. Methods: We included in a prospective, observational and longitudinal study 1194 patients with depressive episode, who were assessed with Clinical Global Impressions Scale (CGI scale) and Quick Inventory of Depressive Symptomatology Scale (QUIDS-C16), in order to perform a correlational analysis. Results: In this study, the target group had a moderate symptomatology (depressed mood, loss of concentration, anhedonia) and had a good evolution from the second week of treatment and a considerable improvement of anhedonia after 10 weeks of treatment. Conclusions: The antidepressant treatment with Agomelatine was considered efficient and safe, with an important improvement of the symptomatology after 10 weeks of treatment, a good treatment adherence and improvement in daily functioning.
1. INTRODUCTION
M a j o r D e p r e s s i v e D i s o r d e r ( M D D ) i s a heterogeneous, complex and debilitating disorder that put a burden not only upon patients but also on their family and society (1). MDD considerably affects patient’s daily functionality with important effect on their quality of life because the social functionality decreases as depression’s severity increases. The literature data are showing that almost 52% of patients with Major Depressive Episode (MDE) have persistent and important symptomatology compared to 18% of patients with mild MDE that experience major difficulties in daily social interactions (2).
Even if the available antidepressants (AD) help patients to obtain a good response or remission of symptoms, there is a period of several weeks until their entire pharmacological action is perceived by patients and clinicians Even so many patients have inadequate response, comorbid symptoms that are not completely controlled or under a poly-pharmacotherapy that can be associated with tolerability issues (1).
There are still significant controversies regarding the moment when the symptomatic amelioration became significant on the evolution of MDE treated with AD. Even though several methodological limitations exist (like the focusing on the clinical response rather than upon symptomatology remission), the results collected from randomized clinical trials (RCT), meta-analyses and observational studies show that: (A). in general AD are associated with early amelioration of the symptoms which can be observed in the first 2 weeks since antidepressant treatment initiation (defined as a reduction ≥ 20% in depression severity measured by standardized rating scales); (B) several antidepressants like Agomelatine are associated with early amelioration of both central symptomatology (depressed mood and anhedonia) and specific symptoms like sleep-wake disturbances; (C) early amelioration is a predictor of symptomatology remission and early response. Subsequently, the lack of amelioration could be seen as a predictor of nonresponse (3).
According to analytical studies on factors and groups there has been shown that depressive mood (negative emotions), anhedonia and psychomotor symptoms are the elements that define major depressive disorders the best (4). Diverse studies showed that anhedonia can precede the onset of a depressive episode, it can influence its severity, can be a predictor of a bad outcome 12 months later and it is a residual symptom frequently present. The inclusion of patients in subtypes according to the type of symptoms and phenomenological approach could constitute a further step of the future psychopharmacology in order to prescribe the best antidepressant according to specific symptoms. There are articles on this subject in the literature but until now the results are not yet conclusive (5).
2. METHODS
2.1 Participants
The study population was constituted of in and outpatients from 59 representative centers in Romania. The investigators were psychiatrists working in the public health system (hospital or specialized ambulatory) or in private practice. The observational study took place
between 1st of February 2012 and 8th of June 2012.
In the study were included 1194 patients with a first MDE or a recurrent MDE according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [6], over 18 years old but under 75 years, whose doctors recommended Agomelatine before the inclusion in the study and who accepted in common agreement with their clinician to be monitored during study. In the final analysis we also included patients with bipolar disorder (2.68%, n=32) and patients without specification of MDE (10%, n=125) due to the fact that this was a naturalistic study and these diagnostics were modified after baseline.
Out of 1194 MDE patients included in the study, more than half (645 patients) were with recurrent MDE. The exclusion criteria referred to the patients that: (A) went to the doctor in emergency regime, (B) use of psychoactive substances, (C) have an age under 18 years or above 75 years old and (D) presented serious comorbidities and/or severe pathology which could affect their participation in the study (hepatic impairment, limited cooperation, legal limited capacity, another severe non-psychiatric disorder, cancer, medication abuse, severe cardiovascular disease or renal failure).
The main objective of this observational study was the evaluation of daily functionality in MDD patients under AD treatment, who were prospectively followed. The secondary objectives included MDE severity improvement and MDD symptomatology evolution in these
patients and the adherence to the AD treatment.
The prospective observation of the patients lasted for 10 weeks and included 4 visits: V0- baseline, V1- 2 weeks since inclusion, V2 and V3 at 6 respectively 10 weeks since inclusion. At the initial visit (baseline) d e p r e s s i v e m o o d , a n h e d o n i a a n d f o c u s i n g difficulties/decision making were the most prominent symptoms as measured with Quick Inventory of Depressive Symptomatology scale.
2.2 Instruments
In order to evaluate the MDE standard instruments were used: CGI scale (Clinical Global Impressions Scale). The CGI scale is an instrument through which the clinician evaluates 3 different global parameters (7):
A. The severity of the disease: global evaluation of actual severity of patient’s symptoms (CGI-S);
B. The global improvement: general comparison between patient’s actual state and the initial (baseline) state (CGI-I)
C. Efficacy index: general comparison between patient’s initial state and a rapport between the actual therapeutic benefit and severity of adverse reactions (CGI-E).
For severity evaluation of MDE Quick Inventory of Depressive Symptomatology scale (QIDS-C16 – Rush et al., 2003) was used. The QIDS16 scale includes all the symptomatology domains, which was developed on the base of DSM-IV criteria for diagnosis of major depressive episode [8,9]: 4 items for evaluation of sleep disturbances;
2 items for evaluation of psychomotor disturbances (agitation or slowing of the psychomotor function); 4 for appetite/ weight evaluation (increasing/decreasing of appetite and weight gain/loss) and only one item for evaluating the other 6 domains (depressed mood, low interest, decreased level of energy, feelings of inutility/ guilt, concentration/decision making difficulties, suicidal ideation). Each item is evaluated on a scale from 0 to 3. For the domains that require the evaluation of least 2 items, the highest score is considered. For example if at the beginning of sleep the value of insomnia is 0, the value of insomnia the middle of the night sleep is 1, for morning insomnia is 3 and 0 for hypersomnia, then the sleep disturbances domain gains a total score of 4 points. The total score varies between 0 and 27. The reference interval in the evaluation of symptoms severity is the 7 days period prior to evaluation. QIDS is sensitive to the change of the clinical state status associated with medical treatment, psychotherapy or somatic treatment, being a very good instrument in both research and clinical practice.
The degree of adherence to the treatment throughout study development was evaluated by the number of pills declared by the patient as taken from one visit to another. study was prospective, observational and longitudinal; all the participants signed an inform consent before being included into the study.
2.3 The statistical analysis was done by CEGEDIM Company using Kynos Modalisa software with a confidence level of 95%, a maximum sampling error for all patients of ± 2.81% and p-value.
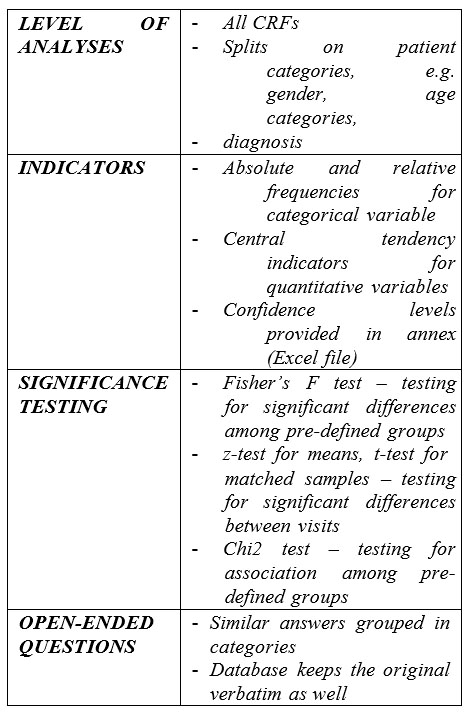
Table 1 – Statistical parameters
3. RESULTS
3.1. Demographic characteristics:
Women represented 68% of the population (n=1190) with a mean age of 48 years. A higher representation had the patients included in the 55 to 60 years’ age group (18%),followed by patients in the 50 to 55 age group (17%)
(n=1186). The lowest representation in the group was for the patients under 25 years (3%). No gender differences were seen regarding the type of episode and the duration of anterior affliction in patients with a current major depressive episode. The characteristics of the study group are presented in Table 2.
3.2. QIDS-C16 score and symptoms evolution: The average of QIDS-C16 score at inclusion was 12.25. This is associated with medium intensity symptoms. The symptoms were ameliorated by the AD treatment reaching a final score of 1.77 at V3 (the interval 0-5 is considered in normal range). The symptoms evolution was statistically significant between visits and also at the final visit (Table
3). The evolution of the symptoms severity at each evaluation (comparing to the initial moment) is graphically presented in Figure 1.
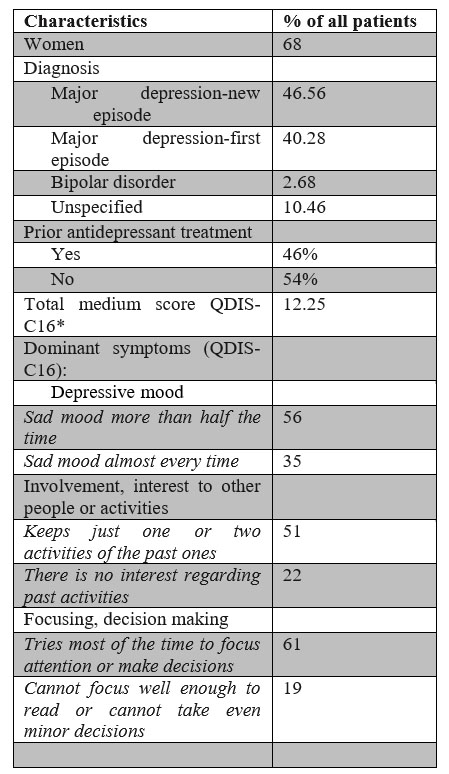
* n=1186-1189 for each domain evaluated with QIDS-C16
Table 2: Baseline characteristics of the MDD patients
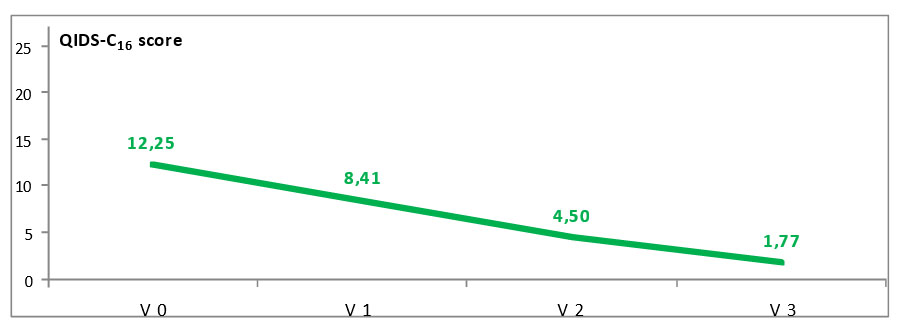
Figure 1: Evolution of the MDE severity during study
(QIDS-C16 scale)
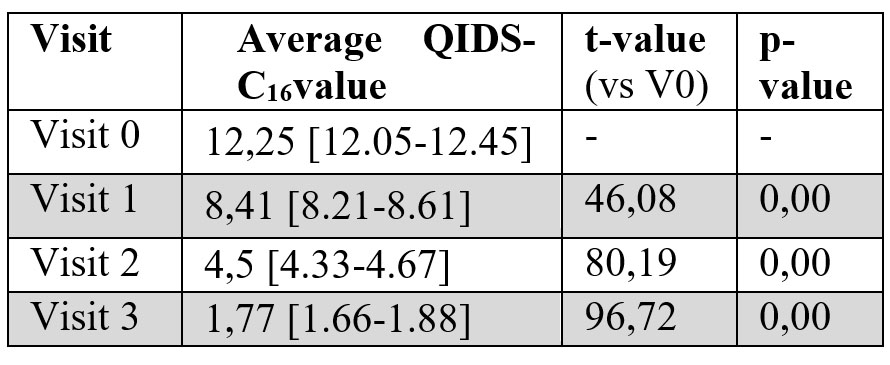
Table 3: Evolution of the MDE symptoms evaluated with QIDS-C16 scale
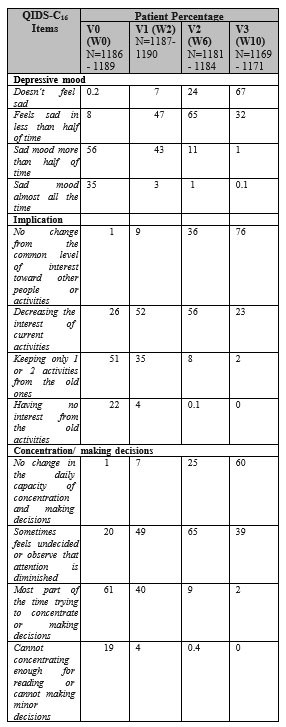
Table 4: The evolution of the depressed mood, anhedonia and impaired concentration/ making decisions during the study (QIDS-C16)
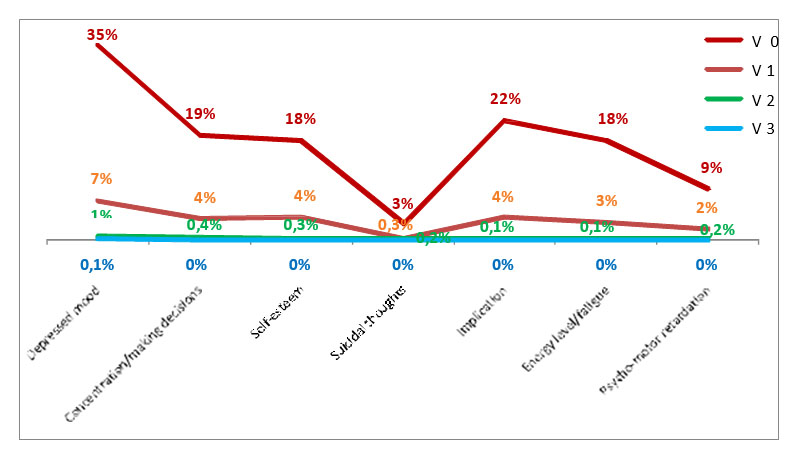
Figure 2: The evolution of the highest score symptomatology on QIDS-C16 scale
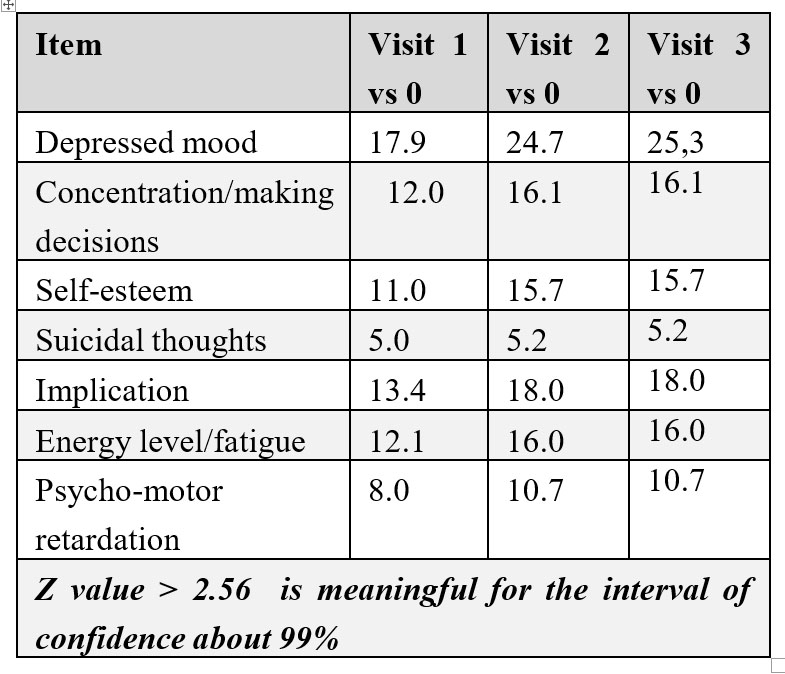
Table 5: Z test applied on the QIDS-C16 items
The statistics revealed that while all the patients presented a significant improvement of the symptomatology, some categories have registered more progress, as follows (supplementary details can be obtained on request):
·Age: The elderly patients (age between 65 and 75) have a slower improvement than other age categories, for all the visits (p=0.002 at V1, p=0.001 at V2 and V3);
·Major depressive episode (new/recurrent): it has been noted significant differences between the patients with a new major depressive episode and recurrent in all that concerns the total scores (ANOVA), but the items scores (Chi2). The patients with recurrent MDD had a slowly recovery.
3.3. Comorbidities of the MDD on the patients of the study:
The presence of somatic comorbidity was observed in
26% of the patients; the most frequently reported comorbidities (n=307) were arterial hypertension (82%) and the ischemic coronary disease (30%).
3.4. Adherence to treatment during the study:
From the patients who had previous AD treatment (n=645, 54%), the main reason for switching/discontinuing was the loss of therapeutic response, followed by the patients’ choice not to be treated with that AD or considering that he does not need treatment anymore. Though, there are cases for which this reason could not be specified.
At the end of the study98.5% of the patients (n-
1194) continued the AD treatment (7 patients discontinued the treatment due to economic reasons).During the observational study there have not been reported any adverse reactions linked to Agomelatine according to the investigators clinical judgement.
4. DISCUSSIONS
We noticed a concordance of the results of this study with the data from the literature regarding the percent of women with MDD and the low prevalence of depression in the age category under 25 years (2).
The group of patients enrolled in this study had a moderate symptomatology and this could be explained by the fact that patients who were addressing to the emergency services were excluded. Another explanation could be the presence of the residual symptoms in patients with recurrent depression and their treatment was changed because of the loss of therapeutic response (the main reason of the changing anterior treatment in this study).
For this group of patients, the symptomatology domains at the initial moments and who had a considerable improvement starting with the second week of treatment have been represented by the depressed mood, loss of concentration and making decisions and anhedonia. Of the three domains, after ten weeks of
treatment anhedonia presented a better improvement. (3rd table). Considering that the medication for the study was represented exclusively by Agomelatine, it is possible that this substance plays an important role which can concord with the results of the recent studies (4, 10).
In this study, the patients recently diagnosed (<3months) and treated have presented a significant improvement after ten weeks of treatment compared with the patients diagnosed with depression more than one year before. Also, the patients with more than 65 years old diagnosed with depression more than one year before had a slow improvement during all the visits. These two observations sustain the importance of recognizing depression and the initiation of the treatment from the beginning, which allow a good improvement of the functionality.
A good adherence to the treatment is a great contributor to the improvement of the symptomatology and thus, to recovering the level of functionality before the disease episode. No side effects related to the AD treatment were recorded according to the clinician’s judgement.
Considering that the medication was based almost exclusively on Agomelatine, the results of the study can be considered representative for this substance. In the same time, we cannot make specific correlations, the Chi test being extremely sensitive in big samples. Another limit can be represented by the short period of the study – 10 weeks, which can permit an observation of the evolution and the adherence only for the acute phase of the treatment. It was observed a good improvement of the functionality from the self-reported data by the patients. At the same time, it might be useful to make a correlation of the functionality with the symptomatology through the evaluation of the patient’s response at a self-administered scale for functionality.
5. CONCLUSIONS
The AD treatment with considered efficient and the MDE patients. After 10 weeks of treatment it has been noticed a good improvement of MDE patients’ symptomatology, a good adherence to treatment and a good improvement of the functionality from the self- reported data by the patients.
6. DISCLOSURE
The authors declare that received in the past grants from Servier Pharma and other speaker fees. In addition, all the authors received in the past speaker’s fees and/or sponsorship from Servier Pharma and others companies for scientific research or communication. Dr. Traian Purnichi also works as a marketing specialist consultant for Servier Pharma and Dr. George Paraschiv and Iulia- Alma Mitea are employees of Servier Pharma.
7. ACKNOWLEDGEMETS
All the authors had an equal contribution and have similar rights. All the authors approved the final version of this article.
The authors will like to thank:
-Cegedim Company that did the statistical analysis using a Kynos Modalisa software.
-MedinteractivPlus for helping on the study draft.
– Servier Pharma for offering the grant that made this study possible.
– The following doctors for assessing the patients included in this study: Ramona Baba, , Alexandrina Baloescu, Ciprian Bacila Magdalena Blaj, RuxandraBloj, Mihaela Brad, Magdalena Bujor, Ion Burlacu, IeronimBurghelea, Ana Campeanu, Liliana Chelariu, Angela Chelcea, R a i s a C i r l i g , Vi c t o r C o j o c a r u , D e l i a C o l t o i u , M i n o d o r a D u m i t r e s c u , C o r i n a – M i h a e l a E c h i m , L i v i u G a n e a , F e l i c i a G i u rg i , C a r m e n H a r a g u s , AncutaHordoan, CameliaHriban, Ioana Ilea, Sorin Iova, Bogdana Lazar, MinodoraManea, Auras Margina, LigiaMincu, Eli Morman, Eugenia ElzaMoruzzi, Eduard Motoescu, Ildiko Must, VioricaNancu, Laura Pacurar- Catana, Corina Panzaru, Ioana Papava, Rita Platona, Alina Pop, Cristian Popa, NeoniliaPopa, Elena Preda, Dan Prelipceanu, Eugenia Roca, Dan Rusu, HoratiuSalvan, Iulia Daria Scorei, Daniela Sichitiu, Lia Sonia Simon, Adina Singalevici, Rodica Stan, Luigya-Claudia Stanci, JeniStanciu, Izabela Stania, AncaTalasman, Livia Taran, Andreea Teodorescu, AlexandruTiugan, Mihaela Tranca, Silvia Trandafir, Corina Tudor, Monica Varlan, Claudiu Ionut Vasile, AndreiaVasilescu, Nicolae Vlad.
8. FINANCIAL SUPORT
This study was founded by a grant from Servier Pharma.
REFERENCES
1.Bodinat C. de, Guardiala-Lemaitre B., Mocaer E., Renard P., Munoz C., Millan MJ.: Agomelatine, the first melatonergic antidepressant: discovery, characterization and development, Nature Reviews, Vol 9, August 2010, 628-642
2.Lam R.W., Mok H.: Depression, Oxford University Press, New York, 2008
3.Lam R.W.: Onset, time course and trajectories of improvement with antidepressants, European Neuropsychopharmacology (2012) 22, S492 – S498
4.Giannantonio M., Martinotti G.: Anhedonia and major depression: the role of agomelatine, European Neuropsychopharmacology (2012) 22, S505 – S510
5.Hasler G., Drevets WC., Manji HK., Charney DS., Discovering endophenotypes for major depression, Neuropsycjopharmacology (2004), 29, 1765 – 1781
6.APA, Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. American Psychiatry Association Press, Washington DC. Text revision. 2000
List of abbreviations:
1. CGI scale – Clinical Global Impressions Scale
2. QUIDS – Quick Inventory of Depressive
Symptomatology Scale
3. MDD – Major Depressive disorder
4. MDE – Major Depressive Episode
5. AD – Antidepressant
6. RCT – Randomized Clinical Trials
7. DSM-IV-TR – Diagnostic and Statistical
Manual of Mental Disorders
***



